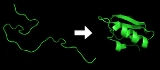
Protein folding
Encyclopedia
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
folds into its characteristic and functional three-dimensional structure
Protein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
from random coil
Random coil
A random coil is a polymer conformation where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the chains in a population of macromolecules...
.
Each protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
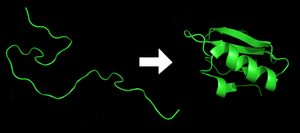
exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. This polypeptide lacks any developed three-dimensional structure (the left hand side of the neighboring figure). Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the native state
Native state
In biochemistry, the native state of a protein is its operative or functional form. While all protein molecules begin as simple unbranched chains of amino acids, once completed they assume highly specific three-dimensional shapes; that ultimate shape, known as tertiary structure, is the folded...
. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma
Anfinsen's dogma
Anfinsen's dogma is a postulate in molecular biology championed by the Nobel Prize Laureate Christian B. Anfinsen...
).
The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded
Intrinsically unstructured proteins
Intrinsically unstructured proteins, often referred to as naturally unfolded proteins or disordered proteins, are proteins characterized by lack of stable tertiary structure when the protein exists as an isolated polypeptide chain under physiological conditions in vitro...
Failure to fold into native structure produces inactive proteins that are usually toxic. Several neurodegenerative and other disease
Disease
A disease is an abnormal condition affecting the body of an organism. It is often construed to be a medical condition associated with specific symptoms and signs. It may be caused by external factors, such as infectious disease, or it may be caused by internal dysfunctions, such as autoimmune...
s are believed to result from the accumulation of amyloid
Amyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
fibrills formed by misfolded proteins. Many allergies are caused by the folding of the proteins, for the immune system does not produce antibodies for certain protein structures.
Relationship between folding and amino acid sequence
Protein biosynthesis
Protein biosynthesis is the process in which cells build or manufacture proteins. The term is sometimes used to refer only to protein translation but more often it refers to a multi-step process, beginning with amino acid synthesis and transcription of nuclear DNA into messenger RNA, which is then...
. While these macromolecule
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
s may be regarded as "folding themselves
Self-assembly
Self-assembly is a term used to describe processes in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction...
", the process also depends on the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
(water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
or lipid bilayer
Lipid bilayer
The lipid bilayer is a thin membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around cells. The cell membrane of almost all living organisms and many viruses are made of a lipid bilayer, as are the membranes surrounding the cell nucleus...
), the concentration of salts, the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, and the presence of molecular chaperones.
Folded proteins usually have a side chain packing stabilizes the folded state, and charged or side chains occupy the solvent-exposed surface where they interact with surrounding water. Minimizing the number of hydrophobic side-chains exposed to water is an important driving force behind the folding process. Formation of intramolecular hydrogen bonds provides another important contribution to protein stability. The strength of hydrogen bonds depends on their environment, thus H-bonds enveloped in a hydrophobic core contribute more than H-bonds exposed to the aqueous environment to the stability of the native state.
The process of folding often begins co-translationally
Translation (genetics)
In molecular biology and genetics, translation is the third stage of protein biosynthesis . In translation, messenger RNA produced by transcription is decoded by the ribosome to produce a specific amino acid chain, or polypeptide, that will later fold into an active protein...
, so that the N-terminus of the protein begins to fold while the C-terminal portion of the protein is still being synthesized
Protein biosynthesis
Protein biosynthesis is the process in which cells build or manufacture proteins. The term is sometimes used to refer only to protein translation but more often it refers to a multi-step process, beginning with amino acid synthesis and transcription of nuclear DNA into messenger RNA, which is then...
by the ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
. Specialized proteins called chaperones assist in the folding of other proteins. A well studied example is the bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
l GroEL
GroEL
GroEL belongs to the chaperonin family of molecular chaperones, and is found in a large number of bacteria. It is required for the proper folding of many proteins. To function properly, GroEL requires the lid-like cochaperonin protein complex GroES...
system, which assists in the folding of globular protein
Globular protein
Globular proteins, or spheroproteins are one of the two main protein classes, comprising "globe"-like proteins that are more or less soluble in aqueous solutions...
s. In eukaryotic organism
Organism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
s chaperones are known as heat shock protein
Heat shock protein
Heat shock proteins are a class of functionally related proteins involved in the folding and unfolding of other proteins. Their expression is increased when cells are exposed to elevated temperatures or other stress. This increase in expression is transcriptionally regulated...
s. Although most globular proteins are able to assume their native state unassisted, chaperone-assisted folding is often necessary in the crowded intracellular environment to prevent aggregation; chaperones are also used to prevent misfolding and aggregation that may occur as a consequence of exposure to heat or other changes in the cellular environment.
There are two models of protein folding that are currently being confirmed:
The first: The diffusion collision model, in which a nucleus is formed, then the secondary structure is formed, and finally these secondary structures are collided together and pack tightly together.
The second: The nucleation-condensation model, in which the secondary and tertiary structures of the protein are made at the same time.
Recent studies have shown that some proteins show characteristics of both of these folding models.
For the most part, scientists have been able to study many identical molecules folding together en masse. At the coarsest level, it appears that in transitioning to the native state, a given amino acid sequence takes on roughly the same route and proceeds through roughly the same intermediates and transition states. Often folding involves first the establishment of regular secondary and supersecondary structures, in particular alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
and beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
s, and afterward tertiary structure. Formation of quaternary structure usually involves the "assembly" or "coassembly" of subunits that have already folded. The regular alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
and beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
structures fold rapidly because they are stabilized by intramolecular hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s, as was first characterized by Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
. Protein folding may involve covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
ing in the form of disulfide bridges
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
formed between two cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues or the formation of metal clusters. Shortly before settling into their more energetically favourable native conformation, molecules may pass through an intermediate "molten globule
Molten globule
The term molten globule was first coined by A. Wada and M Ohgushi in 1983. It was first found in cytochrome c, which conserves a native-like secondary structure content but without the tightly packed protein interior, under low pH and high salt concentration...
" state.
The essential fact of folding, however, remains that the amino acid sequence of each protein contains the information that specifies both the native structure and the pathway to attain that state. This is not to say that nearly identical amino acid sequences always fold similarly. Conformations differ based on environmental factors as well; similar proteins fold differently based on where they are found. Folding is a spontaneous process
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
independent of energy inputs from nucleoside triphosphate
Nucleoside triphosphate
Nucleoside triphosphate is a nucleoside with three phosphates. Natural nucleoside triphosphates include adenosine triphosphate , guanosine triphosphate , cytidine triphosphate , 5-methyluridine triphosphate , and uridine triphosphate . These terms refer to those nucleoside triphosphates that...
s. The passage of the folded state is mainly guided by hydrophobic interactions, formation of intramolecular hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s, and van der Waals forces, and it is opposed by conformational entropy
Conformational entropy
Conformational entropy is the entropy associated with the physical arrangement of a polymer chain that assumes a compact or globular state in solution. The concept is most commonly applied to biological macromolecules such as proteins and RNA, but can also be used for polysaccharides and other...
.
Disruption of the native state
Under some conditions proteins will not fold into their biochemically functional forms. Temperatures above or below the range that cells tend to live in will cause thermally unstableThermostability
Thermostability is the quality of a substance to resist irreversible change in its chemical or physical structure at a high relative temperature.Thermostable materials may be used industrially as fire retardants...
proteins to unfold or "denature
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
" (this is why boiling makes an egg white turn opaque). High concentrations of solutes
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
, extremes of pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
, mechanical forces, and the presence of chemical denaturants can do the same. Protein thermal stability is far from constant, however. For example, hyperthermophilic bacteria have been found that grow at temperatures as high as 122 °C, which of course requires that their full complement of vital proteins and protein assemblies be stable at that temperature or above.
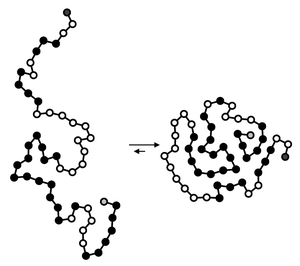
A fully denatured protein lacks both tertiary and secondary structure, and exists as a so-called random coil
Random coil
A random coil is a polymer conformation where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the chains in a population of macromolecules...
. Under certain conditions some proteins can refold; however, in many cases, denaturation is irreversible. Cells sometimes protect their proteins against the denaturing influence of heat with enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s known as chaperones or heat shock protein
Heat shock protein
Heat shock proteins are a class of functionally related proteins involved in the folding and unfolding of other proteins. Their expression is increased when cells are exposed to elevated temperatures or other stress. This increase in expression is transcriptionally regulated...
s, which assist other proteins both in folding and in remaining folded. Some proteins never fold in cells at all except with the assistance of chaperone molecules, which either isolate individual proteins so that their folding is not interrupted by interactions with other proteins or help to unfold misfolded proteins, giving them a second chance to refold properly. This function is crucial to prevent the risk of precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
into insoluble amorphous aggregates.
Incorrect protein folding and neurodegenerative disease
Aggregated proteins are associated with prionPrion
A prion is an infectious agent composed of protein in a misfolded form. This is in contrast to all other known infectious agents which must contain nucleic acids . The word prion, coined in 1982 by Stanley B. Prusiner, is a portmanteau derived from the words protein and infection...
-related illnesses such as Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease
Creutzfeldt–Jakob disease or CJD is a degenerative neurological disorder that is incurable and invariably fatal. CJD is at times called a human form of mad cow disease, given that bovine spongiform encephalopathy is believed to be the cause of variant Creutzfeldt–Jakob disease in humans.CJD...
, bovine spongiform encephalopathy
Bovine spongiform encephalopathy
Bovine spongiform encephalopathy , commonly known as mad-cow disease, is a fatal neurodegenerative disease in cattle that causes a spongy degeneration in the brain and spinal cord. BSE has a long incubation period, about 30 months to 8 years, usually affecting adult cattle at a peak age onset of...
(mad cow disease), amyloid
Amyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
-related illnesses such as Alzheimer's disease
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
and familial amyloid cardiomyopathy or polyneuropathy, as well as intracytoplasmic aggregation diseases such as Huntington's and Parkinson's disease. These age onset degenerative diseases are associated with the aggregation of misfolded proteins into insoluble, extracellular aggregates and/or intracellular inclusions including cross-beta sheet amyloid
Amyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
fibrils. While it is not completely clear whether the aggregates are the cause or merely a reflection of the loss of protein homeostasis, the balance between synthesis, folding, aggregation and protein turnover, the recent European Medicines Agency
European Medicines Agency
The European Medicines Agency is a European agency for the evaluation of medicinal products. From 1995 to 2004, the European Medicines Agency was known as European Agency for the Evaluation of Medicinal Products.Roughly parallel to the U.S...
approval of Tafamidis or Vyndaqel (a kinetic stabilizer of tetrameric transthyretin) for the treatment of the transthyretin amyloid diseases suggests that it is the process of amyloid fibril formation and not the fibrils themselves that causes the degeneration of post-mitotic tissue in human amyloid diseases. Misfolding and excessive degradation instead of folding and function leads to a number of proteopathy
Proteopathy
In medicine, proteopathy refers to a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body...
diseases such as antitrypsin-associated emphysema
Emphysema
Emphysema is a long-term, progressive disease of the lungs that primarily causes shortness of breath. In people with emphysema, the tissues necessary to support the physical shape and function of the lungs are destroyed. It is included in a group of diseases called chronic obstructive pulmonary...
, cystic fibrosis
Cystic fibrosis
Cystic fibrosis is a recessive genetic disease affecting most critically the lungs, and also the pancreas, liver, and intestine...
and the lysosomal storage diseases, where loss of function is the origin of the disorder. While protein replacement therapy has historically been used to correct the latter disorders, an emerging approach is to use pharmaceutical chaperones to fold mutated proteins to render them functional.
Effect of external factors on the folding of Proteins
Several external factors such as temperature, external fields (electric, magnetic), molecular crowding, limitation of space could have a big influence on thefolding of proteins. Modification of the local minima by external
factors can also induce modifications of the folding trajectory.
Protein folding is a very finely tuned process. Hydrogen bonding between different atoms provides the force required. Hydrophobic interactions between hydrophobic amino acids pack the hydrophobic residues
The Levinthal paradox and kinetics
Levinthal's paradox is a thought experiment, also constituting a self-reference in the theory of protein folding. In 1969, Cyrus Levinthal noted that, because of the very large number of degrees of freedom in an unfolded polypeptide chain, the molecule has an astronomical number of possible conformations. An estimate of 3300 or 10143 was made in one of his papers.The Levinthal paradox
Levinthal paradox
Levinthal's paradox is a thought experiment, also constituting a self-reference in the theory of protein folding. In 1969, Cyrus Levinthal noted that, because of the very large number of degrees of freedom in an unfolded polypeptide chain, the molecule has an astronomical number of possible...
observes that if a protein were folded by sequentially sampling of all possible conformations, it would take an astronomical amount of time to do so, even if the conformations were sampled at a rapid rate (on the nanosecond
Nanosecond
A nanosecond is one billionth of a second . One nanosecond is to one second as one second is to 31.7 years.The word nanosecond is formed by the prefix nano and the unit second. Its symbol is ns....
or picosecond
Picosecond
A picosecond is 10−12 of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 001 seconds. A picosecond is to one second as one second is to 31,700 years....
scale). Based upon the observation that proteins fold much faster than this, Levinthal then proposed that a random conformational search does not occur, and the protein must, therefore, fold through a series of meta-stable intermediate states
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
.
The duration of the folding process varies dramatically depending on the protein of interest. When studied outside the cell
In vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
, the slowest folding proteins require many minutes or hours to fold primarily due to proline isomerization, and must pass through a number of intermediate states, like checkpoints, before the process is complete. On the other hand, very small single-domain
Protein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
proteins with lengths of up to a hundred amino acids typically fold in a single step. Time scales of milliseconds are the norm and the very fastest known protein folding reactions are complete within a few microseconds.
Experimental techniques for studying protein folding
While inferences about protein folding can be made through mutation studies; typically, experimental techniques for studying protein folding rely on the gradual unfoldingEquilibrium unfolding
In biochemistry, equilibrium unfolding is the process of unfolding a protein or RNA molecule by gradually changing its environment, such as by changing the temperature or pressure, adding chemical denaturants, or applying force as with an atomic force microscope tip. Since equilibrium is...
or folding of a solution of proteins and observing conformational changes using standard non-crystallographic techniques for observing protein structure.
Protein nuclear magnetic resonance spectroscopy
Protein folding is routinely studied using NMR spectroscopyNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, for example by monitoring hydrogen-deuterium exchange
Hydrogen-deuterium exchange
Hydrogen–deuterium exchange is a chemical reaction in which a covalently bonded hydrogen atom is replaced by a deuterium atom, or vice versa. Usually the examined protons are the amides in the backbone of a protein. The method gives information about the solvent accessibility of various parts of...
of partially folded intermediates.
Circular dichroism
Circular dichroismCircular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
is one of the most general and basic tools to study protein folding. Circular dichroism
Circular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
spectroscopy measures the absorption of circularly polarized light
Circular polarization
In electrodynamics, circular polarization of an electromagnetic wave is a polarization in which the electric field of the passing wave does not change strength but only changes direction in a rotary type manner....
. In proteins, structures such as alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
and beta sheets are chiral, and thus absorb such light. The absorption of this light acts as a marker of the degree of foldedness of the protein ensemble. This technique has been used to measure equilibrium unfolding
Equilibrium unfolding
In biochemistry, equilibrium unfolding is the process of unfolding a protein or RNA molecule by gradually changing its environment, such as by changing the temperature or pressure, adding chemical denaturants, or applying force as with an atomic force microscope tip. Since equilibrium is...
of the protein by measuring the change in this absorption as a function of denaturant
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
concentration or temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. A denaturant
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
melt measures the free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of unfolding as well as the protein's m value, or denaturant dependence. A temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
melt measures the melting temperature
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
(Tm) of the protein. This type of spectroscopy can also be combined with fast-mixing devices, such as stopped flow
Stopped flow
A stopped flow instrument is a rapid mixing device used to study the chemical kinetics of a reaction in solution. After two or more solutions containing the reagents are mixed, they are studied by whatever experimental methods are deemed suitable. Different forms of spectroscopy and scattering of...
, to measure protein folding kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
and to generate chevron plot
Chevron plot
A chevron plot is a way of representing protein folding kinetic data in the presence of varying concentrations of denaturant that disrupts the protein's native tertiary structure...
s.
Dual polarisation interferometry
Dual polarisation interferometryDual Polarisation Interferometry
Dual polarization interferometry is an analytical technique that can probe molecular scale layers adsorbed to the surface of a waveguide by using the evanescent wave of a laser beam confined to the waveguide...
is a surface based technique for measuring the optical properties of molecular layers. When used to characterise protein folding, it measures the conformation by determining the overall size of a monolayer of the protein and its density in real time at sub-Angstrom resolution . Although real time, measurement of the kinetics of protein folding are limited to processes that occur slower than ~10 Hz. Similar to circular dichroism
Circular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
the stimulus for folding can be a denaturant
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
or temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
.
Vibrational circular dichroism of proteins
The more recent developments of vibrational circular dichroismVibrational circular dichroism
Vibrational circular dichroism is a spectroscopic technique which detects differences in attenuation of left and right circularly polarized light passing through a sample...
(VCD) techniques for proteins, currently involving Fourier transform
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
(FFT
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
) instruments, provide powerful means for determining protein conformations in solution even for very large protein molecules. Such VCD studies of proteins are often combined with X-ray diffraction of protein crystals, FT-IR data for protein solutions in heavy water (D2O), or ab initio quantum computations to provide unambiguous structural assignments that are unobtainable from CD
Circular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
.
Studies of folding with high time resolution
The study of protein folding has been greatly advanced in recent years by the development of fast, time-resolved techniques. These are experimental methods for rapidly triggering the folding of a sample of unfolded protein, and then observing the resulting dynamics. Fast techniques in widespread use include neutron scatteringNeutron scattering
Neutron scattering,the scattering of free neutrons by matter,is a physical processand an experimental technique using this processfor the investigation of materials.Neutron scattering as a physical process is of primordial importance...
, ultrafast mixing of solutions, photochemical methods, and laser temperature jump spectroscopy. Among the many scientists who have contributed to the development of these techniques are Jeremy Cook, Heinrich Roder, Harry Gray, Martin Gruebele
Martin Gruebele
Martin Gruebele is a German born American biophysicist and computational biologist who is currently James R...
, Brian Dyer, William Eaton, Sheena Radford, Chris Dobson
Chris Dobson
Christopher Martin "Chris" Dobson, FRS, is a British chemist, John Humphrey Plummer Professor of Chemical and Structural Biology at the University of Cambridge, and Master of St John's College, Cambridge. Dobson's research is largely concerned with protein folding and misfolding.Having completed a...
, Alan Fersht
Alan Fersht
Sir Alan Roy Fersht FRS is a British chemist at the MRC Laboratory of Molecular Biology in Cambridge. He is distinguished for his pioneering work on protein folding.-Biography:...
, Bengt Nölting
Bengt Nölting
Bengt Nölting was a German physicist and biophysicist who pioneered various methods in biophysics and engineering. Achievements include studying biological macromolecules, the development of self-evolving computer programs, and the development new energy technologies...
and Lars Konermann.
Energy landscape of protein folding
The protein folding phenomenon was largely an experimental endeavor until the formulation of an energy landscapeEnergy landscape
In physics, an energy landscape is a mapping of all possible conformations of a molecular entity, or the spatial positions of interacting molecules in a system, and their corresponding energy levels, typically Gibbs free energy, on a two- or three-dimensional Cartesian coordinate system.In...
theory of proteins by Joseph Bryngelson and Peter Wolynes in the late 1980s and early 1990s. This approach introduced the principle of minimal frustration,. This principle says that nature has chosen amino acid sequences
so that the folded state of the protein is very stable. In addition, the undesired
interactions between amino acids along the folding pathway are reduced
making the acquisition of the folded state a very fast process.
Even though nature has reduced the level of frustration in proteins,
some degree of it remains up to now as can be observed in the presence of local
minima in the energy landscape of proteins.
A consequence of these evolutionarily selected sequences is that proteins are generally thought to have globally "funneled energy landscapes" (coined by José Onuchic) that are largely directed toward the native state. This "folding funnel
Folding funnel
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells...
" landscape allows the protein to fold to the native state through any of a large number of pathways and intermediates, rather than being restricted to a single mechanism. The theory is supported by both computational simulations of model proteins
Lattice protein
Lattice proteins are highly simplified computer models of proteins which are used to investigate protein folding.Because proteins are such large molecules, there are severe computational limits on the simulated timescales of their behaviour when modeled in all-atom detail...
and experimental studies, and it has been used to improve methods for protein structure prediction
Protein structure prediction
Protein structure prediction is the prediction of the three-dimensional structure of a protein from its amino acid sequence — that is, the prediction of its secondary, tertiary, and quaternary structure from its primary structure. Structure prediction is fundamentally different from the inverse...
and design
Protein design
Protein design is the design of new protein molecules, either from scratch or by making calculated variations on a known structure. The use of rational design techniques for proteins is a major aspect of protein engineering....
. The description of protein folding by the leveling free-energy landscape is also consistent with the 2nd law of thermodynamics. Physically, thinking of landscapes in terms of visualizable potential or total energy surfaces simply with maxima, saddle points, minima, and funnels, rather like geographic landscapes, is perhaps a little misleading. The relevant description is really a highly dimensional phase space in which manifolds might take a variety of more complicated topological forms.
Modeling of protein folding
De novoDe novo
In general usage, de novo is a Latin expression meaning "from the beginning," "afresh," "anew," "beginning again." It is used in:* De novo transcriptome assembly, the method of creating a transcriptome without a reference genome...
or ab initio
Ab initio
ab initio is a Latin term used in English, meaning from the beginning.ab initio may also refer to:* Ab Initio , a leading ETL Tool Software Company in the field of Data Warehousing.* ab initio quantum chemistry methods...
techniques for computational protein structure prediction
Protein structure prediction
Protein structure prediction is the prediction of the three-dimensional structure of a protein from its amino acid sequence — that is, the prediction of its secondary, tertiary, and quaternary structure from its primary structure. Structure prediction is fundamentally different from the inverse...
are related to, but strictly distinct from experimental studies of protein folding. Molecular Dynamics
Molecular dynamics
Molecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
(MD) is an important tool for studying protein folding and dynamics in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
. First equilibrium folding simulations were done using implicit solvent model and Umbrella Sampling
Umbrella sampling
Umbrella sampling is a technique in computational physics and chemistry, used to improve sampling of a system where ergodicity is hindered by the form of the system's energy landscape. It was first suggested by Torrie and Valleau in 1977...
. Because of computational cost, ab initio MD folding simulations with explicit water are limited to peptides and very small proteins. MD simulations of larger proteins remain restricted to dynamics of the experimental structure or its high-temperature unfolding. In order to simulate long-time folding processes (beyond about 1 microsecond), like folding of small-size proteins (about 50 residues) or larger, some approximations or simplifications in protein models need to be introduced. An approach using reduced protein representation (pseudo-atoms representing groups of atoms are defined) and statistical potential
Statistical potential
In protein structure prediction, a statistical potential or knowledge-based potential is an energy function derived from an analysis of known protein structures in the Protein Data Bank....
are useful in protein structure prediction
Protein structure prediction
Protein structure prediction is the prediction of the three-dimensional structure of a protein from its amino acid sequence — that is, the prediction of its secondary, tertiary, and quaternary structure from its primary structure. Structure prediction is fundamentally different from the inverse...
and modeling of the folding pathways.
There are distributed computing projects which use idle CPU
Idle (CPU)
A computer processor is described as idle when it is not being used by any program.Programs which make use of CPU Idle Time mean that they run at a low priority so as not to impact programs that run at normal priority...
or GPU
Molecular modeling on GPU
Molecular modeling on GPU is the technique of using a graphics processing unit for molecular simulations.In 2007, NVIDIA introduced video cards that could be used not only to show graphics but also for scientific calculations. These cards include many arithmetic units working in parallel...
time of personal computers to solve problems such as protein folding or prediction of protein structure, one prominent example being the Folding@Home
Folding@home
Folding@home is a distributed computing project designed to use spare processing power on personal computers to perform simulations of disease-relevant protein folding and other molecular dynamics, and to improve on the methods of doing so...
project. People can run these programs on their computer or PlayStation 3 to support them.

