
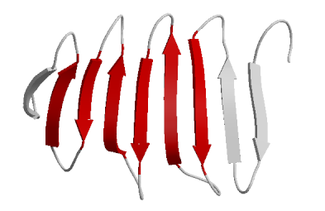
Beta sheet
Encyclopedia
The β sheet is the second form of regular secondary structure
in protein
s, only somewhat less common than the alpha helix
. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bond
s, forming a generally twisted, pleated sheet. A beta strand (also β strand) is a stretch of polypeptide
chain typically 3 to 10 amino acid
s long with backbone in an almost fully extended conformation. The higher-level association of β sheets has been implicated in formation of the protein aggregates and fibrils observed in many human diseases, notably the amyloidoses
such as Alzheimer's disease
.
s to at least one other strand; by contrast, a β sheet refers to an assembly of at least two such β strands that are hydrogen-bonded (or H-bonded) to each other.
in the 1930s. He proposed the idea of hydrogen bonding between the peptide bond
s of parallel or antiparallel extended β strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond
was planar. A refined version was proposed by Linus Pauling
and Robert Corey
in 1951.
network with their neighbors in which the N-H
groups in the backbone of one strand establish hydrogen bond
s with the C=O
groups in the backbone of the adjacent strands. In the fully extended β strand, successive side chains point straight up, then straight down, then straight up, etc. Adjacent β strands in a β sheet are aligned so that their Cα atoms are adjacent and their side chains point in the same direction. The "pleated" appearance of β strands arises from tetrahedral chemical bonding at the Cα atom; for example, if a side chain points straight up, then the bond to the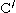 must point slightly downwards, since its bond angle is approximately 109.5°. The pleating causes the distance between
must point slightly downwards, since its bond angle is approximately 109.5°. The pleating causes the distance between  and
and 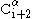 to be approximately 6 Å
to be approximately 6 Å
, rather than the 7.6 Å (2 × 3.8 Å) expected from two fully extended trans peptide
virtual bonds. The "sideways" distance between adjacent Cα atoms in hydrogen-bonded
β strands is roughly 5 Å
However, β strands are rarely perfectly extended; rather, they exhibit a twist due to the chirality
of their component amino acids. The energetically preferred dihedral angle
s near (φ, ψ) = (–135°, 135°) (broadly, the upper left region of the Ramachandran plot
) diverge significantly from the fully extended conformation (φ, ψ) = (–180°, 180°). The twist is often associated with alternating fluctuations in the dihedral angle
s to prevent the individual β strands in a larger sheet from splaying apart. A good example of a strongly twisted β-hairpin can be seen in the protein BPTI.
The side chains point outwards from the folds of the pleats, roughly perpendicularly to the plane of the sheet; successive residues point outwards on alternating faces of the sheet.
and C-terminus
, β strands too can be said to be directional. They are usually represented in protein topology diagrams by an arrow pointing toward the C-terminus. Adjacent β strands can form hydrogen bond
s in antiparallel, parallel, or mixed arrangements.
In an antiparallel arrangement, the successive β strands alternate directions so that the N-terminus of one strand is adjacent to the C-terminus of the next. This is the arrangement that produces the strongest inter-strand stability because it allows the inter-strand hydrogen bonds between carbonyls and amines to be planar, which is their preferred orientation. The peptide backbone dihedral angles (φ, ψ) are about (–140°, 135°) in antiparallel sheets. In this case, if two atoms and
and  are adjacent in two hydrogen-bonded
are adjacent in two hydrogen-bonded
β strands, then they form two mutual backbone hydrogen bonds to each other's flanking peptide groups
; this is known as a close pair of hydrogen bonds.
In a parallel arrangement, all of the N-termini of successive strands are oriented in the same direction; this orientation may be slightly less stable because it introduces nonplanarity in the inter-strand hydrogen bonding pattern. The dihedral angles (φ, ψ) are about (–120°, 115°) in parallel sheets. It is rare to find less than five interacting parallel strands in a motif, suggesting that a smaller number of strands may be unstable, however it is also fundamentally more difficult for parallel β-sheets to form because strands with N and C termini aligned necessarily must be very distant in sequence. There is also evidence that parallel β-sheet may be more stable since small amyloidogenic sequences appear to generally aggregate into β-sheet fibrils composed of primarily parallel β-sheet strands, where one would expect anti-parallel fibrils if anti-parallel was more stable.
In parallel β-sheet structure, if two atoms and
and 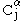 are adjacent in two hydrogen-bonded
are adjacent in two hydrogen-bonded
β strands, then they do not hydrogen bond to each other; rather, one residue forms hydrogen bonds to the residues that flank the other (but not vice versa). For example, residue may form hydrogen bonds to residues
may form hydrogen bonds to residues 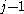 and
and 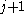 ; this is known as a wide pair of hydrogen bonds. By contrast, residue
; this is known as a wide pair of hydrogen bonds. By contrast, residue 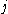 may hydrogen-bond to different residues altogether, or to none at all.
may hydrogen-bond to different residues altogether, or to none at all.
Finally, an individual strand may exhibit a mixed bonding pattern, with a parallel strand on one side and an antiparallel strand on the other. Such arrangements are less common than a random distribution of orientations would suggest, suggesting that this pattern is less stable than the anti-parallel arrangement, however bioinformatic analysis always struggles with extracting structural thermodynamics since there are always numerous other structural features present in whole proteins. Also proteins are inherently constrained by folding kinetics as well as folding thermodynamics, so one must always be careful in concluding stability from bioinformatic analysis.
The hydrogen bond
ing of β strands need not be perfect, but can exhibit localized disruptions known as beta bulge
s.
The hydrogen bonds lie roughly in the plane of the sheet, with the peptide
carbonyl
groups pointing in alternating directions with successive residues; for comparison, successive carbonyls point in the same direction in the alpha helix
.
formation. It is also suggested that the dipole moments in parallel β-sheets, whose direction is from C-terminal (partially negative) to N-terminal (partially positive) may influence the propensity of certain residues (like Lys and Arg) for the caps of this structure.
 A very simple structural motif
A very simple structural motif
involving β sheets is the β hairpin
, in which two antiparallel strands are linked by a short loop of two to five residues, of which one is frequently a glycine
or a proline
, both of which can assume the unusual dihedral-angle conformations required for a tight turn
. However, individual strands can also be linked in more elaborate ways with long loops that may contain alpha helices
or even entire protein domains.
, the TIM barrel
.
protein topology composed of 2 or more consecutive antiparallel β-strands linked together by hairpin
loops. This motif is common in β-sheets and can be found in several structural architectures including β-barrels
and β-propellers.

s or small proteins with poorly defined overall architecture. All-β domains may form β barrels
, β sandwiches, β prisms, β propellers
, and β-helices
.
β strands along the backbone. For example, the flavodoxin fold
has a five-stranded, parallel β sheet with topology 21345; thus, the edge strands are β strand 2 and β strand 5 along the backbone. Spelled out explicitly, β strand 2 is H-bonded to β strand 1, which is H-bonded to β strand 3, which is H-bonded to β strand 4, which is H-bonded to β strand 5, the other edge strand. In the same system, the Greek key motif described above has a 4123 topology. The secondary structure
of a β sheet can be described roughly by giving the number of strands, their topology, and whether their hydrogen bond
s are parallel or antiparallel.
β sheets can be open, meaning that they have two edge strands (as in the flavodoxin fold
or the immunoglobulin fold) or they can be closed beta barrels (such as the TIM barrel
). β-Barrels are often described by their stagger or shear. Some open β sheets are very curved and fold over on themselves (as in the SH3 domain
) or form horseshoe shapes (as in the ribonuclease inhibitor
). Open β sheets can assemble face-to-face (such as the beta-propeller domain
or immunoglobulin fold) or edge-to-edge, forming one big β sheet.
and analyzed with the quasi-continuum model.
is formed from repeating structural units consisting of two or three short β strands linked by short loops. These units "stack" atop one another in a helical fashion so that successive repetitions of the same strand hydrogen-bond with each other in a parallel orientation. See the beta helix
article for further information.
In lefthanded β helices, the strands themselves are quite straight and untwisted; the resulting helical surfaces are nearly flat, forming a regular triangular prism
shape, as shown for the 1QRE archaeal carbonic anhydrase at right. Other examples are the lipid A synthesis enzyme LpxA and insect antifreeze proteins with a regular array of Thr sidechains on one face that mimic the structure of ice.
Righthanded β helices, typified by the pectate lyase
enzyme shown at left or P22 phage
tailspike protein, have a less regular cross-section, longer and indented on one of the sides; of the three linker loops, one is consistently just two residues long and the others are variable, often elaborated to form a binding or active site.
A two-sided β helix (right-handed) is found in some bacterial metalloproteases; its two loops are each six residues long and bind stabilizing calcium ions to maintain the integrity of the structure, using the backbone and the Asp side chain oxygens of a GGXGXD sequence motif. This fold is called a beta-roll in the SCOP classification.
. Its structure has yet to be determined in full, but recent data suggest that it may resemble an unusual two-strand β helix.
The side chains from the amino acid residues found in a β sheet structure may also be arranged such that many of the adjacent sidechains on one side of the sheet are hydrophobic, while many of those adjacent to each other on the alternate side of the sheet are polar or charged (hydrophilic), which can be useful if the sheet is to form a boundary between polar/watery and nonpolar/greasy environments.
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
in protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, only somewhat less common than the alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s, forming a generally twisted, pleated sheet. A beta strand (also β strand) is a stretch of polypeptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
chain typically 3 to 10 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s long with backbone in an almost fully extended conformation. The higher-level association of β sheets has been implicated in formation of the protein aggregates and fibrils observed in many human diseases, notably the amyloidoses
Amyloidosis
In medicine, amyloidosis refers to a variety of conditions whereby the body produces "bad proteins", denoted as amyloid proteins, which are abnormally deposited in organs and/or tissues and cause harm. A protein is described as being amyloid if, due to an alteration in its secondary structure, it...
such as Alzheimer's disease
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
.
Nomenclature
In the most common usage, β strand refers to a single continuous stretch of amino acids adopting an extended conformation and involved in backbone hydrogen bondHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s to at least one other strand; by contrast, a β sheet refers to an assembly of at least two such β strands that are hydrogen-bonded (or H-bonded) to each other.
History
The first β sheet structure was proposed by William AstburyWilliam Astbury
William Thomas Astbury FRS was an English physicist and molecular biologist who made pioneering X-ray diffraction studies of biological molecules. His work on keratin provided the foundation for Linus Pauling's discovery of the alpha helix...
in the 1930s. He proposed the idea of hydrogen bonding between the peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s of parallel or antiparallel extended β strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
was planar. A refined version was proposed by Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
and Robert Corey
Robert Corey
Robert Brainard Corey was an American biochemist, mostly known for his role in discovery of the α-helix and the β-sheet with Linus Pauling. Also working with Pauling was Herman Branson. Their discoveries were remarkably correct, with even the bond lengths being accurate until about 40 years later...
in 1951.
Structure and orientation
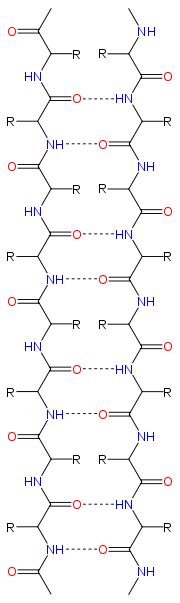 |
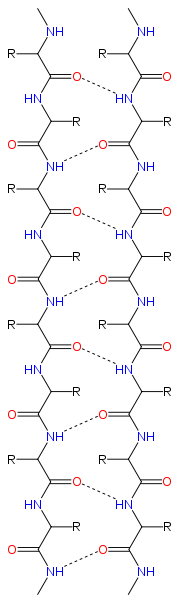 |
Geometry
The majority of β strands are arranged adjacent to other strands and form an extensive hydrogen bondHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
network with their neighbors in which the N-H
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
groups in the backbone of one strand establish hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s with the C=O
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups in the backbone of the adjacent strands. In the fully extended β strand, successive side chains point straight up, then straight down, then straight up, etc. Adjacent β strands in a β sheet are aligned so that their Cα atoms are adjacent and their side chains point in the same direction. The "pleated" appearance of β strands arises from tetrahedral chemical bonding at the Cα atom; for example, if a side chain points straight up, then the bond to the
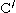 must point slightly downwards, since its bond angle is approximately 109.5°. The pleating causes the distance between
must point slightly downwards, since its bond angle is approximately 109.5°. The pleating causes the distance between  and
and 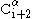 to be approximately 6 Å
to be approximately 6 ÅÅngström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
, rather than the 7.6 Å (2 × 3.8 Å) expected from two fully extended trans peptide
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
virtual bonds. The "sideways" distance between adjacent Cα atoms in hydrogen-bonded
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
β strands is roughly 5 Å
However, β strands are rarely perfectly extended; rather, they exhibit a twist due to the chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
of their component amino acids. The energetically preferred dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s near (φ, ψ) = (–135°, 135°) (broadly, the upper left region of the Ramachandran plot
Ramachandran plot
-Introduction and early history:A Ramachandran plot , originally developed in 1963 by G. N. Ramachandran C. Ramakrishnan and V...
) diverge significantly from the fully extended conformation (φ, ψ) = (–180°, 180°). The twist is often associated with alternating fluctuations in the dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s to prevent the individual β strands in a larger sheet from splaying apart. A good example of a strongly twisted β-hairpin can be seen in the protein BPTI.
The side chains point outwards from the folds of the pleats, roughly perpendicularly to the plane of the sheet; successive residues point outwards on alternating faces of the sheet.
Hydrogen bonding patterns
Because peptide chains have a directionality conferred by their N-terminusN-terminal end
The N-terminus refers to the start of a protein or polypeptide terminated by an amino acid with a free amine group . The convention for writing peptide sequences is to put the N-terminus on the left and write the sequence from N- to C-terminus...
and C-terminus
C-terminal end
The C-terminus is the end of an amino acid chain , terminated by a free carboxyl group . When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus...
, β strands too can be said to be directional. They are usually represented in protein topology diagrams by an arrow pointing toward the C-terminus. Adjacent β strands can form hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s in antiparallel, parallel, or mixed arrangements.
In an antiparallel arrangement, the successive β strands alternate directions so that the N-terminus of one strand is adjacent to the C-terminus of the next. This is the arrangement that produces the strongest inter-strand stability because it allows the inter-strand hydrogen bonds between carbonyls and amines to be planar, which is their preferred orientation. The peptide backbone dihedral angles (φ, ψ) are about (–140°, 135°) in antiparallel sheets. In this case, if two atoms
 and
and  are adjacent in two hydrogen-bonded
are adjacent in two hydrogen-bondedHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
β strands, then they form two mutual backbone hydrogen bonds to each other's flanking peptide groups
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
; this is known as a close pair of hydrogen bonds.
In a parallel arrangement, all of the N-termini of successive strands are oriented in the same direction; this orientation may be slightly less stable because it introduces nonplanarity in the inter-strand hydrogen bonding pattern. The dihedral angles (φ, ψ) are about (–120°, 115°) in parallel sheets. It is rare to find less than five interacting parallel strands in a motif, suggesting that a smaller number of strands may be unstable, however it is also fundamentally more difficult for parallel β-sheets to form because strands with N and C termini aligned necessarily must be very distant in sequence. There is also evidence that parallel β-sheet may be more stable since small amyloidogenic sequences appear to generally aggregate into β-sheet fibrils composed of primarily parallel β-sheet strands, where one would expect anti-parallel fibrils if anti-parallel was more stable.
In parallel β-sheet structure, if two atoms
 and
and 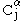 are adjacent in two hydrogen-bonded
are adjacent in two hydrogen-bondedHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
β strands, then they do not hydrogen bond to each other; rather, one residue forms hydrogen bonds to the residues that flank the other (but not vice versa). For example, residue
 may form hydrogen bonds to residues
may form hydrogen bonds to residues 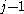 and
and 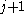 ; this is known as a wide pair of hydrogen bonds. By contrast, residue
; this is known as a wide pair of hydrogen bonds. By contrast, residue 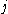 may hydrogen-bond to different residues altogether, or to none at all.
may hydrogen-bond to different residues altogether, or to none at all.Finally, an individual strand may exhibit a mixed bonding pattern, with a parallel strand on one side and an antiparallel strand on the other. Such arrangements are less common than a random distribution of orientations would suggest, suggesting that this pattern is less stable than the anti-parallel arrangement, however bioinformatic analysis always struggles with extracting structural thermodynamics since there are always numerous other structural features present in whole proteins. Also proteins are inherently constrained by folding kinetics as well as folding thermodynamics, so one must always be careful in concluding stability from bioinformatic analysis.
The hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing of β strands need not be perfect, but can exhibit localized disruptions known as beta bulge
Beta bulge
A beta bulge is a localized disruption of the regular hydrogen bonding of a beta sheet, usually by inserting a residue with helical dihedral angles into one or both H-bonded β-strands.-Types:...
s.
The hydrogen bonds lie roughly in the plane of the sheet, with the peptide
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups pointing in alternating directions with successive residues; for comparison, successive carbonyls point in the same direction in the alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
.
Amino acid propensities
Large aromatic residues (Tyr, Phe and Trp) and β-branched amino acids (Thr, Val, Ile) are favored to be found in β strands in the middle of β sheets. Interestingly, different types of residues (such as Pro) are likely to be found in the edge strands in β sheets, presumably to avoid the "edge-to-edge" association between proteins that might lead to aggregation and amyloidAmyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
formation. It is also suggested that the dipole moments in parallel β-sheets, whose direction is from C-terminal (partially negative) to N-terminal (partially positive) may influence the propensity of certain residues (like Lys and Arg) for the caps of this structure.
Common structural motifs

Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
involving β sheets is the β hairpin
Beta hairpin
The beta hairpin structural motif is the simplest protein motif involving two beta strands that look like a hairpin. The motif consists of two strands that are adjacent in primary structure oriented in an antiparallel arrangement and linked by a short loop of two to five amino acids...
, in which two antiparallel strands are linked by a short loop of two to five residues, of which one is frequently a glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
or a proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
, both of which can assume the unusual dihedral-angle conformations required for a tight turn
Turn (biochemistry)
A turn is an element of secondary structure in proteins where the polypeptide chain reverses its overall direction.- Definition :According to the most common definition, a turn is a structural motif where the Cα atoms of two residues separated by few peptide bonds are in close approach A turn is...
. However, individual strands can also be linked in more elaborate ways with long loops that may contain alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
or even entire protein domains.
Greek key motif
The Greek key motif consists of four adjacent antiparallel strands and their linking loops. It consists of three antiparallel strands connected by hairpins, while the fourth is adjacent to the first and linked to the third by a longer loop. This type of structure forms easily during the protein folding process. It was named after a pattern common to Greek ornamental artwork (see meander (art)).The β-α-β motif
Due to the chirality of their component amino acids, all strands exhibit a "right-handed" twist evident in most higher-order β sheet structures. In particular, the linking loop between two parallel strands almost always has a right-handed crossover chirality, which is strongly favored by the inherent twist of the sheet. This linking loop frequently contains a helical region, in which case it is called a β-α-β motif. A closely related motif called a β-α-β-α motif forms the basic component of the most commonly observed protein tertiary structureTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
, the TIM barrel
TIM barrel
The TIM barrel is a conserved protein fold consisting of eight α-helices and eight parallel β-strands that alternate along the peptide backbone. The structure is named after triosephosphate isomerase, a conserved glycolytic enzyme. TIM barrels are quite common among the conserved protein folds...
.

β-meander motif
A simple supersecondaryStructural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
protein topology composed of 2 or more consecutive antiparallel β-strands linked together by hairpin
Beta hairpin
The beta hairpin structural motif is the simplest protein motif involving two beta strands that look like a hairpin. The motif consists of two strands that are adjacent in primary structure oriented in an antiparallel arrangement and linked by a short loop of two to five amino acids...
loops. This motif is common in β-sheets and can be found in several structural architectures including β-barrels
Beta barrel
A beta barrel is a large beta-sheet that twists and coils to form a closed structure in which the first strand is hydrogen bonded to the last.Beta-strands in beta-barrels are typically arranged in an antiparallel fashion...
and β-propellers.

Psi-loop motif
The psi-loop, Ψ-loop, motif consists of two antiparallel strands with one strand in between that is connected to both by hydrogen bonds. There are four possible strand topologies for single Ψ-loops as cited by Hutchinson et al. (1990). This motif is rare as the process resulting in its formation seems unlikely to occur during protein folding. The Ψ-loop was first identified in the aspartic protease family.Structural architectures of proteins with beta-sheets
Beta-sheets are present in all-β, α+β and α/β domains according to Structural Classification of Proteins and in many peptidePeptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s or small proteins with poorly defined overall architecture. All-β domains may form β barrels
Beta barrel
A beta barrel is a large beta-sheet that twists and coils to form a closed structure in which the first strand is hydrogen bonded to the last.Beta-strands in beta-barrels are typically arranged in an antiparallel fashion...
, β sandwiches, β prisms, β propellers
Beta-propeller domain
A beta-propeller is a type of all-β protein architecture characterized by 4 to 8 blade-shaped beta sheets arranged toroidally around a central axis. Each sheet typically has four antiparallel β-strands twisted so that the first and fourth strands are almost perpendicular to each other...
, and β-helices
Beta helix
A beta helix is a protein structure formed by the association of parallel beta strands in a helical pattern with either two or three faces. The structure is stabilized by inter-strand hydrogen bonds, protein-protein interactions, and sometimes bound metal ions...
.
Structural topology
The topology of a β sheet describes the order of hydrogen-bondedHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
β strands along the backbone. For example, the flavodoxin fold
Flavodoxin fold
thumb|left|300px|Ribbon diagram of CheY , which adopts the flavodoxin fold. Ribbon is colored from blue to red ....
has a five-stranded, parallel β sheet with topology 21345; thus, the edge strands are β strand 2 and β strand 5 along the backbone. Spelled out explicitly, β strand 2 is H-bonded to β strand 1, which is H-bonded to β strand 3, which is H-bonded to β strand 4, which is H-bonded to β strand 5, the other edge strand. In the same system, the Greek key motif described above has a 4123 topology. The secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
of a β sheet can be described roughly by giving the number of strands, their topology, and whether their hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s are parallel or antiparallel.
β sheets can be open, meaning that they have two edge strands (as in the flavodoxin fold
Flavodoxin fold
thumb|left|300px|Ribbon diagram of CheY , which adopts the flavodoxin fold. Ribbon is colored from blue to red ....
or the immunoglobulin fold) or they can be closed beta barrels (such as the TIM barrel
TIM barrel
The TIM barrel is a conserved protein fold consisting of eight α-helices and eight parallel β-strands that alternate along the peptide backbone. The structure is named after triosephosphate isomerase, a conserved glycolytic enzyme. TIM barrels are quite common among the conserved protein folds...
). β-Barrels are often described by their stagger or shear. Some open β sheets are very curved and fold over on themselves (as in the SH3 domain
SH3 domain
The SRC Homology 3 Domain is a small protein domain of about 60 amino acids residues first identified as a conserved sequence in the viral adaptor protein v-Crk and the non-catalytic parts of enzymes such as phospholipase and several cytoplasmic tyrosine kinases such as Abl and Src...
) or form horseshoe shapes (as in the ribonuclease inhibitor
Ribonuclease inhibitor
Ribonuclease inhibitor is a large , acidic , leucine-rich repeat protein that forms extremely tight complexes with certain ribonucleases. It is a major cellular protein, comprising ~0.1% of all cellular protein by weight, and appears to play an important role in regulating the lifetime of RNA.RI...
). Open β sheets can assemble face-to-face (such as the beta-propeller domain
Beta-propeller domain
A beta-propeller is a type of all-β protein architecture characterized by 4 to 8 blade-shaped beta sheets arranged toroidally around a central axis. Each sheet typically has four antiparallel β-strands twisted so that the first and fourth strands are almost perpendicular to each other...
or immunoglobulin fold) or edge-to-edge, forming one big β sheet.
Dynamic features
Beta-sheets in proteins may carry out low-frequency accordion-like motion as observed by the Raman spectroscopyRaman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
and analyzed with the quasi-continuum model.
Parallel β helices
A β helixBeta helix
A beta helix is a protein structure formed by the association of parallel beta strands in a helical pattern with either two or three faces. The structure is stabilized by inter-strand hydrogen bonds, protein-protein interactions, and sometimes bound metal ions...
is formed from repeating structural units consisting of two or three short β strands linked by short loops. These units "stack" atop one another in a helical fashion so that successive repetitions of the same strand hydrogen-bond with each other in a parallel orientation. See the beta helix
Beta helix
A beta helix is a protein structure formed by the association of parallel beta strands in a helical pattern with either two or three faces. The structure is stabilized by inter-strand hydrogen bonds, protein-protein interactions, and sometimes bound metal ions...
article for further information.
In lefthanded β helices, the strands themselves are quite straight and untwisted; the resulting helical surfaces are nearly flat, forming a regular triangular prism
Triangular prism
In geometry, a triangular prism is a three-sided prism; it is a polyhedron made of a triangular base, a translated copy, and 3 faces joining corresponding sides....
shape, as shown for the 1QRE archaeal carbonic anhydrase at right. Other examples are the lipid A synthesis enzyme LpxA and insect antifreeze proteins with a regular array of Thr sidechains on one face that mimic the structure of ice.
Righthanded β helices, typified by the pectate lyase
Pectate lyase
Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. Pectate lyase is responsible for the eliminative cleavage of pectate, yielding oligosaccharides with 4-deoxy-alpha-D-mann-4-enuronosyl groups at their non-reducing ends. The protein is maximally expressed late...
enzyme shown at left or P22 phage
P22 phage
The P22 virus is a lysogenic bacteriophage that infects Salmonella. Its linear, double-stranded DNA genome is 44 kilobases long. Like many phage viruses, it has been used in molecular biology to induce mutations in cultured bacteria and to introduce foreign genetic material. The viral capsid has...
tailspike protein, have a less regular cross-section, longer and indented on one of the sides; of the three linker loops, one is consistently just two residues long and the others are variable, often elaborated to form a binding or active site.
A two-sided β helix (right-handed) is found in some bacterial metalloproteases; its two loops are each six residues long and bind stabilizing calcium ions to maintain the integrity of the structure, using the backbone and the Asp side chain oxygens of a GGXGXD sequence motif. This fold is called a beta-roll in the SCOP classification.
β sheets in pathology
Some proteins that are disordered or helical as monomers, such as amyloid β (see amyloid plaque) can form β-sheet-rich oligomeric structures associated with pathological states. The amyloid β protein's oligomeric form is implicated as a cause of Alzheimer'sAlzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
. Its structure has yet to be determined in full, but recent data suggest that it may resemble an unusual two-strand β helix.
The side chains from the amino acid residues found in a β sheet structure may also be arranged such that many of the adjacent sidechains on one side of the sheet are hydrophobic, while many of those adjacent to each other on the alternate side of the sheet are polar or charged (hydrophilic), which can be useful if the sheet is to form a boundary between polar/watery and nonpolar/greasy environments.
See also
- Folding (chemistry)Folding (chemistry)In chemistry, folding is the process by which a molecule assumes its shape or conformation. The process can also be described as intramolecular self-assembly where the molecule is directed to form a specific shape through noncovalent interactions, such as hydrogen bonding, metal coordination,...
- Tertiary structureTertiary structureIn biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
- α helixAlpha helixA common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
- Collagen helixCollagen helixIn collagen, the collagen helix, or type-2 helix, is a major shape in secondary structure. It consists of a triple helix made of the repetitious amino acid sequence glycine - X - Y, where X and Y are frequently proline or hydroxyproline....
- FoldamersFoldamersA foldamer, is a discrete chain molecule or oligomer that adopts a secondary structure stabilized by noncovalent interactions. They are artificial molecules that mimic the ability of proteins, nucleic acids, and polysaccharides to fold into well-defined conformations, such as helices and β-sheets...
Further reading
- Cooper, J. "Super Secondary Structure - Part II", May 31, 1996. Accessed May 25, 2007.
- Structural Classification of Proteins (SCOP) "Open-sided Beta-meander", October 20, 2006. Accessed May 31, 2007.

