Circular dichroism
Overview
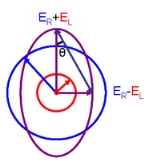
Circular polarization
In electrodynamics, circular polarization of an electromagnetic wave is a polarization in which the electric field of the passing wave does not change strength but only changes direction in a rotary type manner....
light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
. This phenomenon was discovered by Jean-Baptiste Biot
Jean-Baptiste Biot
Jean-Baptiste Biot was a French physicist, astronomer, and mathematician who established the reality of meteorites, made an early balloon flight, and studied the polarization of light.- Biography :...
, Augustin Fresnel, and Aimé Cotton
Aimé Cotton
Aimé Auguste Cotton was a French physicist known for his studies of the interaction of light with chiral molecules...
in the first half of the 19th century. It is exhibited in the absorption bands
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
of optically active chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
molecules. CD spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
has a wide range of applications in many different fields. Most notably, UV
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
CD is used to investigate the secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
of proteins. UV/Vis CD is used to investigate charge-transfer transitions.

