
Alpha helix
Encyclopedia
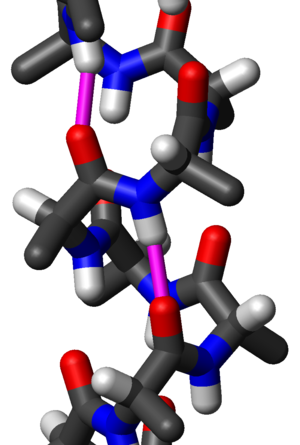
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, the alpha helix (α-helix) is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
to the backbone C=O
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group of the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
four residues
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
earlier (
 hydrogen bonding). This secondary structure is also sometimes called a classic Pauling–Corey–Branson alpha helix (see below). Among types of local structure in proteins, the α-helix is the most regular and the most predictable from sequence, as well as the most prevalent.
hydrogen bonding). This secondary structure is also sometimes called a classic Pauling–Corey–Branson alpha helix (see below). Among types of local structure in proteins, the α-helix is the most regular and the most predictable from sequence, as well as the most prevalent.Historical development
In the early 1930s, William AstburyWilliam Astbury
William Thomas Astbury FRS was an English physicist and molecular biologist who made pioneering X-ray diffraction studies of biological molecules. His work on keratin provided the foundation for Linus Pauling's discovery of the alpha helix...
showed that there were drastic changes in the X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
fiber diffraction
Fiber diffraction
Fiber diffraction is a subarea of scattering, an area in which molecular structure is determined from scattering data . In fiber diffraction the scattering pattern does not change, as the sample is rotated about a unique axis...
of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ~5.1 ångström (0.51 nm).
Astbury initially proposed a kinked-chain structure for the fibers. He later joined other researchers (notably the American chemist Maurice Huggins) in proposing that:
- the unstretched protein molecules formed a helix (which he called the α-form); and
- the stretching caused the helix to uncoil, forming an extended state (which he called the β-form).
Although incorrect in their details, Astbury's models of these forms were correct in essence and correspond to modern elements of secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
, the α-helix and the β-strand
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
(Astbury's nomenclature was kept), which were developed by Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
, Robert Corey
Robert Corey
Robert Brainard Corey was an American biochemist, mostly known for his role in discovery of the α-helix and the β-sheet with Linus Pauling. Also working with Pauling was Herman Branson. Their discoveries were remarkably correct, with even the bond lengths being accurate until about 40 years later...
and Herman Branson
Herman Branson
Herman Russell Branson was an African-American physicist, best known for his research on the alpha helix protein structure, and was also the president of two colleges.- Early life :...
in 1951 (see below); that paper showed both right- and left-handed helices, although in 1960 the crystal structure of myoglobin showed that the right-handed form is the common one. Hans Neurath
Hans Neurath
Hans Neurath was a biochemist, a leader in protein chemistry and the founding chairman of the Department of Biochemistry at the University of Washington in Seattle.-Early life:...
was the first to show that Astbury's models could not be correct in detail, because they involved clashes of atoms. Neurath's paper and Astbury's data inspired H. S. Taylor
Hugh Stott Taylor
Hugh Stott Taylor was an English chemist primarily interested in catalysis. In 1928, in a landmark contribution to catalytic theory, Taylor suggested that a catalyzed chemical reaction is not catalyzed over the entire solid surface of the catalyst but only at certain ‘active sites’ or centers.He...
, Maurice Huggins
Maurice Loyal Huggins
Maurice Loyal Huggins was a scientist who independently conceived the idea of hydrogen bonding and who was an early advocate for their role in stabilizing protein secondary structure...
and Bragg
William Lawrence Bragg
Sir William Lawrence Bragg CH OBE MC FRS was an Australian-born British physicist and X-ray crystallographer, discoverer of the Bragg law of X-ray diffraction, which is basic for the determination of crystal structure. He was joint winner of the Nobel Prize for Physics in 1915. He was knighted...
and collaborators to propose models of keratin
Keratin
Keratin refers to a family of fibrous structural proteins. Keratin is the key of structural material making up the outer layer of human skin. It is also the key structural component of hair and nails...
that somewhat resemble the modern α-helix.
Two key developments in the modeling of the modern α-helix were (1) the correct bond geometry, thanks to the crystal structure determinations
Crystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s and peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s and Pauling's prediction of planar peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s; and (2) his relinquishing of the assumption of an integral number of residues per turn of the helix. The pivotal moment came in the early spring of 1948, when Pauling caught a cold and went to bed. Being bored, he drew a polypeptide chain of roughly correct dimensions on a strip of paper and folded it into a helix, being careful to maintain the planar peptide bonds. After a few attempts, he produced a model with physically plausible hydrogen bonds. Pauling then worked with Corey and Branson to confirm his model before publication. In 1954 Pauling was awarded his first Nobel Prize "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances"http://nobelprize.org/nobel_prizes/chemistry/laureates/1954/ (such as proteins), prominently including the structure of the α-helix.
Geometry and hydrogen bonding
The amino acids in an α helix are arranged in a right-handed helicalHelix
A helix is a type of smooth space curve, i.e. a curve in three-dimensional space. It has the property that the tangent line at any point makes a constant angle with a fixed line called the axis. Examples of helixes are coil springs and the handrails of spiral staircases. A "filled-in" helix – for...
structure where each amino acid residue corresponds to a 100° turn in the helix (i.e., the helix has 3.6 residues per turn), and a translation of 1.5 Å (0.15 nm) along the helical axis. Dunitz describes how Pauling's first article on the theme in fact shows a left-handed helix, the enantiomer of the true structure. Short pieces of left-handed helix sometimes occur with a large content of achiral glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
amino acids, but are unfavorable for the other normal, biological L-amino acids. The pitch of the alpha-helix (the vertical distance between one consecutive turn of the helix) is 5.4 Å (0.54 nm) which is the product of 1.5 and 3.6. What is most important is that the N-H
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group of an amino acid forms a hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
with the C=O
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group of the amino acid four residues earlier; this repeated
 hydrogen bonding is the most prominent characteristic of an α-helix. Official international nomenclature http://www.chem.qmul.ac.uk/iupac/misc/ppep1.html specifies two ways of defining α-helices, rule 6.2 in terms of repeating φ,ψ torsion angles (see below) and rule 6.3 in terms of the combined pattern of pitch and hydrogen bonding. The alpha-helices can be identified in protein structure using several computational methods, one of which is DSSP
hydrogen bonding is the most prominent characteristic of an α-helix. Official international nomenclature http://www.chem.qmul.ac.uk/iupac/misc/ppep1.html specifies two ways of defining α-helices, rule 6.2 in terms of repeating φ,ψ torsion angles (see below) and rule 6.3 in terms of the combined pattern of pitch and hydrogen bonding. The alpha-helices can be identified in protein structure using several computational methods, one of which is DSSPDSSP (protein)
The DSSP algorithm is the standard method for assigning secondary structure to the amino acids of a protein, given the atomic-resolution coordinates of the protein...
(Dictionary of Protein Secondary Structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
).
Similar structures include the 310 helix (
 hydrogen bonding) and the π-helix (
hydrogen bonding) and the π-helix (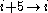 hydrogen bonding). The α helix can be described as a 3.613 helix, since the i + 4 spacing adds 3 more atoms to the H-bonded loop compared to the tighter 310 helix. The subscripts refer to the number of atoms (including the hydrogen) in the closed loop formed by the hydrogen bond.
hydrogen bonding). The α helix can be described as a 3.613 helix, since the i + 4 spacing adds 3 more atoms to the H-bonded loop compared to the tighter 310 helix. The subscripts refer to the number of atoms (including the hydrogen) in the closed loop formed by the hydrogen bond.Residues in α-helices typically adopt backbone (φ, ψ) dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s around (-60°, -45°), as shown in the image at right. In more general terms, they adopt dihedral angles such that the ψ dihedral angle of one residue and the φ dihedral angle of the next residue sum to roughly -105°. As a consequence, α-helical dihedral angles, in general, fall on a diagonal stripe on the Ramachandran diagram (of slope -1), ranging from (-90°, -15°) to (-35°, -70°). For comparison, the sum of the dihedral angles for a 310 helix is roughly -75°, whereas that for the π-helix is roughly -130°. The general formula for the rotation angle Ω per residue of any polypeptide helix with trans isomers is given by the equation
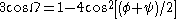
The α-helix is tightly packed; there is almost no free space within the helix. The amino-acid side chains are on the outside of the helix, and point roughly "downwards" (i.e., towards the N-terminus), like the branches of an evergreen tree (Christmas tree
Christmas tree
The Christmas tree is a decorated evergreen coniferous tree, real or artificial, and a tradition associated with the celebration of Christmas. The tradition of decorating an evergreen tree at Christmas started in Livonia and Germany in the 16th century...
effect). This directionality is sometimes used in preliminary, low-resolution electron-density maps to determine the direction of the protein backbone.
2D (dimensional) diagrams for representing alpha-helices
Two different kinds of 2D diagrams are usually used to represent α-helices. One is called the “helical wheelHelical wheel
A helical wheel is a type of plot or visual representation used to illustrate the properties of alpha helices in proteins. The sequence of amino acids that make up a helical region of the protein's secondary structure are plotted in a rotating manner where the angle of rotation between consecutive...
”, and the other called “wenxiang diagram”. The latter name came from the fact that it looks like a coil-like incense used in China to repel mosquitos; Chinese 蚊香 http://wapedia.mobi/zh/%E8%9A%8A%E9%A6%99 .
In the wenxiang diagram each amino acid residue is represented by a circle with a letter to indicate its single character code: a hydrophobic residue is denoted by a filled circle with a white code symbol, whereas a hydrophilic residue is denoted by an open circle with a black code symbol. As a 2D representation, the wenxiang diagram has the following features: (1) able to show the relative locations of the amino acids in an alpha-helix regardless how long it is; (2) able to indicate the direction of an alpha-helix; and (3) having the capacity to provide more information about each of the constituent amino acid residues in an α-helix.
With these features, the wenxiang diagram can provides an intuitive and easily visualizable picture in a 2D space that characterizes the disposition of hydrophobic and hydrophilic residues in α-helices.
Wenxiang diagrams have been used to study helix-helix interactions. Wenxiang diagrams are particularly useful to help gain insights into the interactions between proteins that contain amphiphilic helices.
Stability
Helices observed in proteins can range from four to over forty residues long, but a typical helix contains about ten amino acids (about three turns). In general, short polypeptides do not exhibit much alpha helical structure in solution, since the entropicEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
cost associated with the folding of the polypeptide chain is not compensated for by a sufficient amount of stabilizing interactions. In general, the backbone hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s of α-helices are considered slightly weaker than those found in β-sheets
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
, and are readily attacked by the ambient water molecules. However, in more hydrophobic environments such as the plasma membrane, or in the presence of co-solvents such as trifluoroethanol (TFE), or isolated from solvent in the gas phase, oligopeptides readily adopt stable α-helical structure. Furthermore, crosslinks can be incorporated into peptides to conformationally stabilize helical folds. Crosslinks stabilize the helical state by entropically destabilizing the unfolded state and by removing enthalpically stabilized "decoy" folds that compete with the fully helical state.
Experimental determination
Since the α-helix is defined by its hydrogen bonds and backbone conformation, the most detailed experimental evidence for α-helical structure comes from atomic-resolution X-ray crystallographyX-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
such as the example shown at right. It is clear that all the backbone carbonyl oxygens point downward (toward the C-terminus) but splay out slightly, and the H-bonds are approximately parallel to the helix axis. Protein structures from NMR spectroscopy also show helices well, with characteristic observations of NOE (Nuclear Overhauser Effect
Nuclear Overhauser effect
The Nuclear Overhauser Effect is the transfer of nuclear spin polarization from one nuclear spin population to another via cross-relaxation. It is a common phenomenon observed by nuclear magnetic resonance spectroscopy. The theoretical basis for the NOE was described and experimentally verified...
) couplings between atoms on adjacent helical turns. In some cases, the individual hydrogen bonds can be observed directly as a small scalar coupling in NMR.
There are several lower-resolution methods for assigning general helical structure. The NMR chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
s (in particular of the
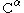 ,
, 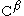 and
and  atoms) and residual dipolar coupling
atoms) and residual dipolar couplingResidual dipolar coupling
The residual dipolar coupling between two spins in a molecule occurs if the molecules in solution exhibit a partial alignment leading to an incomplete averaging of spatially anisotropic dipolar couplings....
s are often characteristic of helices. The far-UV (170-250 nm) circular dichroism
Circular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
spectrum of helices is also idiosyncratic, exhibiting a pronounced double minimum at ~208 nm and ~222 nm. Infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
spectroscopy is rarely used, since the α-helical spectrum resembles that of a random coil
Random coil
A random coil is a polymer conformation where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the chains in a population of macromolecules...
(although these might be discerned by, e.g., hydrogen-deuterium exchange
Hydrogen-deuterium exchange
Hydrogen–deuterium exchange is a chemical reaction in which a covalently bonded hydrogen atom is replaced by a deuterium atom, or vice versa. Usually the examined protons are the amides in the backbone of a protein. The method gives information about the solvent accessibility of various parts of...
). Finally, cryo electron microscopy is now capable of discerning individual α-helices within a protein, although their assignment to residues is still an active area of research.
Long homopolymers of amino acids often form helices if soluble. Such long, isolated helices can also be detected by other methods, such as dielectric relaxation, flow birefringence
Flow birefringence
In biochemistry, flow birefringence is a hydrodynamic technique for measuring the rotational diffusion constants . The birefringence of a solution sandwiched between two concentric cylinders is measured as a function of the difference in rotational speed between the inner and outer cylinders...
and measurements of the diffusion constant. In stricter terms, these methods detect only the characteristic prolate (long cigar-like) hydrodynamic shape of a helix, or its large dipole moment.
Amino-acid propensities
Different amino-acid sequences have different propensities for forming α-helical structure. MethionineMethionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
, alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
, leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
, uncharged glutamate, and lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
("MALEK" in the amino-acid 1-letter codes) all have especially high helix-forming propensities, whereas proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
and glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
have poor helix-forming propensities. Proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
either breaks or kinks a helix, both because it cannot donate an amide hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
(having no amide hydrogen), and also because its sidechain interferes sterically with the backbone of the preceding turn - inside a helix, this forces a bend of about 30° in the helix axis. However, proline is often seen as the first residue of a helix, presumably due to its structural rigidity. At the other extreme, glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
also tends to disrupt helices because its high conformational flexibility makes it entropically expensive to adopt the relatively constrained α-helical structure.
Dipole moment
A helix has an overall dipole moment caused by the aggregate effect of all the individual dipoles from the carbonylCarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups of the peptide bond pointing along the helix axis. This can lead to destabilization of the helix through entropic effects. As a result, α helices are often capped at the N-terminal end by a negatively charged amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
, such as glutamic acid
Glutamic acid
Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates...
, in order to Neutralize this helix dipole. Less common (and less effective) is C-terminal capping with a positively charged amino acid, such as lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
. The N-terminal positive charge is commonly used to bind negatively charged ligands such as phosphate groups, which is especially effective because the backbone amides can serve as hydrogen bond donors.
Larger-scale assemblies
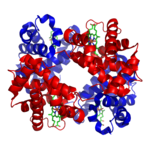
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
and hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
, the first two proteins whose structures were solved by X-ray crystallography
Crystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
, have very similar folds made up of about 70% α helix, with the rest being non-repetitive regions, or "loops" which connect the helices. In classifying proteins by their dominant fold, the Structural Classification of Proteins database maintains a large category specifically for all-α proteins.
Coiled-coil α helices are highly stable forms in which two or more helices wrap around each other in a "supercoil" structure. Coiled coil
Coiled coil
A coiled coil is a structural motif in proteins, in which 2-7 alpha-helices are coiled together like the strands of a rope . Many coiled coil type proteins are involved in important biological functions such as the regulation of gene expression e.g. transcription factors...
s contain a highly characteristic sequence motif known as a heptad repeat
Heptad repeat
The heptad repeat is an example of a structural motif that consists of a repeating pattern of seven amino acids: a b c d e f g H P P H C P C...
, in which the motif repeats itself every seven residues along the sequence. The first and especially the fourth residues (known as the a and d positions) are almost always hydrophobic (the fourth residue is typically leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
) and pack together in the interior of the helix bundle. In general, the fifth and seventh residues (the e and g positions) have opposing charges and form a salt bridge stabilized by electrostatic interactions. Fibrous protein
Fibrous protein
Scleroproteins, or fibrous proteins, constitute one of the three main classes of proteins, alongside globular proteins and conjugated proteins.Keratin, collagen, elastin, and fibroin are all scleroproteins...
s such as keratin and myosin often adopt coiled-coil structures, as do several dimerizing proteins. A pair of coiled-coils - a four-helix bundle
Helix bundle
A helix bundle is a small protein fold composed of several alpha helices that are usually nearly parallel or antiparallel to each other.-Three-helix bundles:Three-helix bundles are among the smallest and fastest known cooperatively folding structural domains...
- is a very common structural motif in proteins. For example, it occurs in human growth hormone
Growth hormone
Growth hormone is a peptide hormone that stimulates growth, cell reproduction and regeneration in humans and other animals. Growth hormone is a 191-amino acid, single-chain polypeptide that is synthesized, stored, and secreted by the somatotroph cells within the lateral wings of the anterior...
and several varieties of cytochrome
Cytochrome
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
. The Rop protein, which promotes plasmid replication in bacteria, is an interesting case in which a single polypeptide forms a coiled-coil and two monomers assemble to form a four-helix bundle.
The amino acids that make up a particular helix can be plotted on a helical wheel
Helical wheel
A helical wheel is a type of plot or visual representation used to illustrate the properties of alpha helices in proteins. The sequence of amino acids that make up a helical region of the protein's secondary structure are plotted in a rotating manner where the angle of rotation between consecutive...
, a representation that illustrates the orientations of the constituent amino acids. Often in globular protein
Globular protein
Globular proteins, or spheroproteins are one of the two main protein classes, comprising "globe"-like proteins that are more or less soluble in aqueous solutions...
s, as well as in specialized structures such as coiled-coils and leucine zipper
Leucine zipper
A leucine zipper, aka leucine scissors, is a common three-dimensional structural motif in proteins. These motifs are usually found as part of a DNA-binding domain in various transcription factors, and are therefore involved in regulating gene expression...
s, an alpha helix will exhibit two "faces" - one containing predominantly hydrophobic amino acids oriented toward the interior of the protein, in the hydrophobic core, and one containing predominantly polar amino acids oriented toward the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
-exposed surface of the protein.
Functional roles
DNA binding
α-helices have particular significance in DNADNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
binding motifs, including helix-turn-helix
Helix-turn-helix
In proteins, the helix-turn-helix is a major structural motif capable of binding DNA. It is composed of two α helices joined by a short strand of amino acids and is found in many proteins that regulate gene expression...
motifs, leucine zipper
Leucine zipper
A leucine zipper, aka leucine scissors, is a common three-dimensional structural motif in proteins. These motifs are usually found as part of a DNA-binding domain in various transcription factors, and are therefore involved in regulating gene expression...
motifs and zinc finger
Zinc finger
Zinc fingers are small protein structural motifs that can coordinate one or more zinc ions to help stabilize their folds. They can be classified into several different structural families and typically function as interaction modules that bind DNA, RNA, proteins, or small molecules...
motifs. This is because of the convenient structural fact that the diameter of the α helix is 1.2 nanometres, the same as the width of the major groove in B-form DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
, and also because coiled-coil (or leucine zipper) dimers of helices can readily position a pair of interaction surfaces to contact the sort of symmetrical repeat common in double-helical DNA (see Branden & Tooze, chapter 10). An example of both aspects is the transcription factor
Transcription factor
In molecular biology and genetics, a transcription factor is a protein that binds to specific DNA sequences, thereby controlling the flow of genetic information from DNA to mRNA...
Max (see image at left), which uses a helical coiled-coil to dimerize, positioning another pair of helices for interaction in two successive turns of the DNA major groove.
Membrane spanning
α-helices are also the most common protein structure element that crosses biological membranes (see Branden & Tooze, chapter 12), it is presumed because the helical structure can satisfy all backbone hydrogen-bonds internally, leaving no polar groups exposed to the membrane if the sidechains are hydrophobic. Proteins are sometimes anchored by a single membrane-spanning helix, sometimes by a pair, and sometimes by a helix bundle, most classically consisting of seven helices arranged up-and-down in a ring such as for rhodopsinRhodopsin
Rhodopsin, also known as visual purple, is a biological pigment of the retina that is responsible for both the formation of the photoreceptor cells and the first events in the perception of light. Rhodopsins belong to the G-protein coupled receptor family and are extremely sensitive to light,...
s (see image at right) or for G protein–coupled receptors (GPCRs).
Mechanical properties
α-helices under axial tensile deformation, a characteristic loading condition that appears in many alpha-helix rich filaments and tissues, results in a characteristic three-phase behavior of stiff-soft-stiff tangent modulus. Phase I corresponds to the small-deformation regime during which the helix is stretched homogeneously, followed by phase II in which alpha-helical turns break mediated by the rupture of groups of H-bonds. Phase III is typically associated with large-deformation covalent bond stretching.Dynamical features
Alpha-helices in proteins may have low-frequency accordion-like motion as observed by the Raman spectroscopyRaman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
and analyzed via the quasi-continuum model.
Helix-coil transition
Homopolymers of amino-acids (such as poly-lysine) can adopt α-helical structure at low temperature that is "melted out" at high temperatures. This helix-coil transition was once thought to be analogous to protein denaturationDenaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
. The statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
of this transition can be modeled using an elegant transfer matrix
Transfer matrix
The transfer matrix is a formulation in terms of a block-Toeplitz matrix of the two-scale equation, which characterizes refinable functions. Refinable functions play an important role in wavelet theory and finite element theory....
method, characterized by two parameters: the propensity to initiate a helix and the propensity to extend a helix.
The α-helix in art
At least three artists have made explicit reference to the α-helix in their work, Julie Newdoll in painting and Julian Voss-AndreaeJulian Voss-Andreae
Julian Voss-Andreae is a German sculptor living and working in the U.S.Voss-Andreae started out as a painter and later studied experimental physics at the universities of Berlin, Edinburgh and Vienna...
and Bathsheba Grossman
Bathsheba Grossman
Bathsheba Grossman is an artist in Santa Cruz, California who creates sculptures using computer-aided design and three-dimensional modeling, with metal printing technology to produce sculpture in bronze and stainless steel. Her bronze sculptures are primarily mathematical in nature, often...
in sculpture.
San Francisco area artist Julie Newdoll http://www.newdoll.com/, who holds a degree in Microbiology, and a minor in art, has specialized in paintings inspired by microscopic images and molecules since 1990. Her painting "Rise of the Alpha Helix" (2003) features human figures arranged in an α helical arrangement. According to the artist, "the flowers reflect the various types of sidechains that each amino acid holds out to the world"http://www.newdoll.com/. It is interesting to note that this same metaphor is also echoed from the scientist's side: "β sheets do not show a stiff repetitious regularity but flow in graceful, twisting curves, and even the α-helix is regular more in the manner of a flower stem, whose branching nodes show the influence of environment, developmental history, and the evolution of each part to match its own idiosyncratic function."
Julian Voss-Andreae
Julian Voss-Andreae
Julian Voss-Andreae is a German sculptor living and working in the U.S.Voss-Andreae started out as a painter and later studied experimental physics at the universities of Berlin, Edinburgh and Vienna...
is a German-born sculptor with degrees in experimental physics and sculpture. Since 2001 Voss-Andreae creates "protein sculptures" based on protein structure with the α-helix being one of his preferred objects. Voss-Andreae has made α-helix sculptures from diverse materials including bamboo and whole trees. A monument Voss-Andreae created in 2004 to celebrate the memory of Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
, the discoverer of the α-helix, is fashioned from a large steel beam rearranged in the structure of the α-helix. The 10 feet (3 m) tall, bright-red sculpture stands in front of Pauling's childhood home in Portland, Oregon
Portland, Oregon
Portland is a city located in the Pacific Northwest, near the confluence of the Willamette and Columbia rivers in the U.S. state of Oregon. As of the 2010 Census, it had a population of 583,776, making it the 29th most populous city in the United States...
.
Ribbon diagrams of α-helices are a prominent element in the laser-etched crystal sculptures of protein structures created by Bathsheba Grossman
Bathsheba Grossman
Bathsheba Grossman is an artist in Santa Cruz, California who creates sculptures using computer-aided design and three-dimensional modeling, with metal printing technology to produce sculpture in bronze and stainless steel. Her bronze sculptures are primarily mathematical in nature, often...
http://www.bathsheba.com, such as those of insulin
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
, hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
, and DNA polymerase
DNA polymerase
A DNA polymerase is an enzyme that helps catalyze in the polymerization of deoxyribonucleotides into a DNA strand. DNA polymerases are best known for their feedback role in DNA replication, in which the polymerase "reads" an intact DNA strand as a template and uses it to synthesize the new strand....
.
See also
- Folding (chemistry)Folding (chemistry)In chemistry, folding is the process by which a molecule assumes its shape or conformation. The process can also be described as intramolecular self-assembly where the molecule is directed to form a specific shape through noncovalent interactions, such as hydrogen bonding, metal coordination,...
- Beta sheetBeta sheetThe β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
- Knobs into holes packingKnobs into holes packingKnobs into holes packing is a protein packing motif that occurs mainly in alpha helix or coiled coil domains. One such example is fibrinogen fibril formation....
- Secondary structureSecondary structureIn biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
- Tertiary structureTertiary structureIn biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
- Davydov solitonDavydov solitonDavydov soliton is a quantum quasiparticle representing an excitation propagating along the protein α-helix self-trapped amide I. It is a solution of the Davydov Hamiltonian. It is named for the Soviet and Ukrainian physicist Alexander Davydov. The Davydov model describes the interaction of the...

