Fullerene chemistry
Encyclopedia
Fullerene chemistry is a field of organic chemistry
devoted to the chemical properties of fullerene
s. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes
with trapped molecules inside the cage.
or Ih with 12 pentagons and 20 hexagons. According to Euler's theorem
these 12 pentagons are required for closure of the carbon network consisting of n hexagons and C60 is the first stable fullerene because it is the smallest possible to obey this rule. In this structure none of the pentagons make contact with each other. Both C60 and its relative C70 obey this so-called isolated pentagon rule (IPR). The next homologue C84 has 24 IPR isomers of which several are isolated and another 51,568 non-IPR isomers. Non-IPR fullerenes have thus far only been isolated as endohedral fullerenes such as Tb3N@C84 with two fused pentagons at the apex of an egg-shaped cage. or as fullerenes with exohedral stabilization such as C50Cl10 and reportedly C60H8.
Because of the molecule's spherical shape the carbon atoms are highly pyramidalized, which has far-reaching consequences for reactivity. It is estimated that strain energy
constitutes 80% of the heat of formation. The conjugated carbon atoms respond to deviation from planarity by orbital rehybridization of the sp² orbital
s and pi orbitals to a sp2.27 orbital with a gain in p-character. The p lobes extend further outside the surface than they do into the interior of the sphere and this is one of the reasons a fullerene is electronegative. The other reason is that the empty low-lying pi* orbitals also have high s character.
The double bonds in fullerene are not all the same. Two groups can be identified: 30 so-called [6,6] double bonds connect two hexagons and 60 [5,6] bonds connect a hexagon and a pentagon. Of the two the [6,6] bonds are shorter with more double-bond character and therefore a hexagon is often represented as a cyclohexatriene and a pentagon as a pentalene or [5]radialene
. In other words, although the carbon atoms in fullerene are all conjugated the superstructure is not a super aromatic compound. The X-ray diffraction bond length
values are 135.5 pm for the [6,6] bond and 146.7 pm for the [5,6] bond.
C60 fullerene has 60 pi electrons but a closed shell configuration requires 72 electrons. The fullerene is able to acquire the missing electrons by reaction with potassium
to form first the salt and then the In this compound the bond length alternation observed in the parent molecule has vanished.
when double bonds become saturated. Key in this type of reaction is the level of functionalization i.e. monoaddition or multiple additions and in case of multiple additions their topological relationships (new substituents huddled together or evenly spaced). In conformity with IUPAC rules, the terms methanofullerene are used to indicate the ring-closed (cyclopropane
) fullerene
derivatives, and fulleroid to ring-open (methanoannulene) structures.
s with a host of nucleophiles in nucleophilic addition
s. The intermediary formed carbanion
is captured by another electrophile. Examples of nucleophiles are Grignard reagents and organolithium reagent
s. For example the reaction of C60 with methylmagnesium chloride
stops quantitatively at the penta-adduct with the methyl groups centered around a cyclopentadienyl anion which is subsequently protonated. Another nucleophilic reaction is the Bingel reaction
.
Fullerene reacts with chlorobenzene
and aluminium chloride
in a Friedel-Crafts alkylation type reaction. In this hydroarylation the reaction product is the 1,2-addition adduct (Ar-CC-H).
s for instance Diels-Alder reaction
s. 4-membered rings can be obtained by [2+2]cycloadditions for instance with benzyne. An example of a 1,3-dipolar cycloaddition
to a 5-membered ring is the Prato reaction
. Fullerenes react with carbene
s to methanofullerenes.
and potassium nitrate
to C60(OH)15. Another method is reaction in diluted sodium hydroxide catalysed by TBAH adding 24 to 26 hydroxyl groups. Hydroxylation has also been reported using solvent-free NaOH / hydrogen peroxide
. C60(OH)8 was prepared using a multistep procedure starting from a mixed peroxide fullerene. The maximum number of hydroxyl
groups that can be attached (hydrogen peroxide method) stands at 36-40.
s as well. The reaction with bromine
can add up to 24 bromine atoms to the sphere. The record holder for fluorine addition is C60F48. According to in silico
predictions the as yet elusive C60F60 may have some of the fluorine atoms in endo positions (pointing inwards) and may resemble a tube more than it does a sphere.
in organometallic chemistry
. The [6,6] double bond is electron-deficient and usually forms metallic bonds with η = 2 hapticity
. Bonding modes such as η = 5 or η = 6 can be induced by modification of the coordination sphere
.
of the compound starting from simple organic compounds.
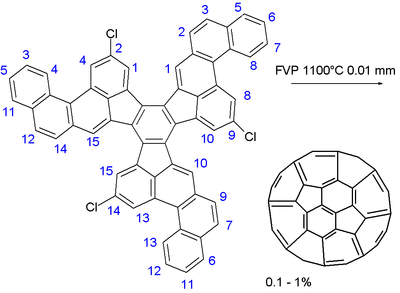
In the final step a large polycyclic aromatic hydrocarbon
consisting of 13 hexagons and three pentagons is submitted to flash vacuum pyrolysis at 1100°C and 0.01 Torr
. The three carbon chlorine bonds serve as free radical incubators and the ball is stitched up in a no-doubt complex series of radical reactions. The chemical yield is low: 0.1 to 1%. A small percentage of fullerenes is formed in any process which involves burning of hydrocarbons, e.g. in candle burning. The yield through a combustion method is often above 1%. The method proposed above does not provide any advantage for synthesis of fullerenes compared to the usual combustion method, and therefore, the organic synthesis of fullerenes remains a challenge for chemistry.
A similar exercise aimed at construction of a C78 cage in 2008 (but leaving out the precursor's halogens) did not result in a sufficient yield but at least the introduction of Stone Wales defect
s could be ruled out.
a bottom-up approach has also been investigated
s the opening, hydrogen insertion and closing back up has already been demonstrated.
to a C120 bucky dumbbell in the solid state by mechanochemistry
(high-speed vibration milling) with potassium cyanide
as a catalyst. The trimer has also been reported using 4-aminopyridine
as catalyst (4% yield) and observed with Scanning Tunneling Microscopy as a monolayer
s, also part of the fullerene family, can be described as graphene
sheets rolled into a cylindrical tube. Unlike the spherical fullerenes made up of hexagons and pentagons, nanotubes only have hexagons present but in terms of reactivity both systems have much in common. Due to electrostatic forces nanotubes have a nasty tendency to cluster together into bundles and many potential applications require an exfoliation process. One way to do this is by chemical surface modification.
A useful tool for the analysis of derivatised nanotubes is Raman spectroscopy
which shows a G-band (G for graphite
) for the native nanotubes at 1580 cm−1 and a D-band (D for defect) at 1280 cm−1 when the graphite lattice is disrupted with conversion of sp² to sp³ hybridized carbon. The ratio of both peaks ID/IG is taken as a measure of functionalization. Other tools are UV spectroscopy where pristine nanotubes show distinct Van Hove singularities
where functionalized tubes do not, and simple TGA analysis
.
In one type of chemical modification, aniline
is oxidized to a diazonium intermediate. After expulsion of nitrogen, it forms a covalent bond as an aryl radical
:
 Also known are protocols for cycloadditions such as Diels-Alder reaction
Also known are protocols for cycloadditions such as Diels-Alder reaction
s , 1,3-dipolar cycloadditions of
azomethine ylides and azide–alkyne cycloaddition reactions. One example is a DA reaction assisted by chromium hexacarbonyl and high pressure. The ID/IG ratio for reaction with Danishefsky’s diene
is 2.6.
Nanotubes can also be alkylated with alkyl halides using lithium or sodium metal and liquid ammonia (Birch reduction
conditions). The initial nanotube salt can function as an polymerization initiator and can react with peroxides to form alkoxy functionalized nanotubes
compound free of contamination. In fullerene production mixtures of C60, C70 and higher homologues are always formed. Fullerene purification is key to fullerene
science and determines fullerene prices and the success of practical applications of fullerenes. The first available purification method for C60 fullerene was by HPLC
from which small amounts could be generated at large expense.
A practical laboratory-scale method for purification of soot enriched in C60 and C70 starts with extraction in toluene
followed by filtration
with a paper filter. The solvent is evaporated and the residue (the toluene-soluble soot fraction) redissolved in toluene and subjected to column chromatography
. C60 elutes first with a purple color and C70 is next displaying a reddish-brown color.
In nanotube processing the established purification method for removing amorphous carbon and metals is by competitive oxidation (often a sulfuric acid
/ nitric acid
mixture). It is assumed that this oxidation creates oxygen containing groups (hydroxyl
, carbonyl
, carboxyl) on the nanotube surface which electrostatically stabilize them in water and which can later be utilized in chemical functionalization. One report reveals that the oxygen containing groups in actuality combine with carbon contaminations absorbed to the nanotube wall that can be removed by a simple base wash. Cleaned nanotubes are reported to have reduced D/G ratio indicative of less functionalization, and the absence of oxygen is also apparent from IR spectroscopy and X-ray photoelectron spectroscopy
.
compound DBU
to a solution of the mixture in 1,2,3-trimethylbenzene
. DBU as it turns out only reacts to C70 fullerenes and higher which reaction products separate out and can be removed by filtration. C60 fullerenes do not have any affinity for DBU and are subsequently isolated. Other diamine compounds like DABCO
do not share this selectivity.
C60 but not C70 forms a 1:2 inclusion compound
with cyclodextrin
(CD). A separation method for both fullerenes based on this principle is made possible by anchoring cyclodextrin to colloidal gold
particles through a sulfur
-sulfur bridge. The Au/CD compound is very stable and soluble in water and selectively extracts C60 from the insoluble mixture after reflux
ing for several days. The C70 fullerene component is then removed by simple filtration
. C60 is driven out from the Au/CD compound by adding adamantol
which has a higher affinity for the cyclodextrin cavity. Au/CD is completely recycled
when adamantol in turn is driven out by adding ethanol
and ethanol removed by evaporation. 50 mg of Au/CD captures 5 mg of C60 fullerene.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
devoted to the chemical properties of fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
s. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes
Endohedral fullerenes
Endohedral fullerenes are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex was synthesized in 1985 called La@C60. The @ sign in the name reflects the notion of a small molecule trapped inside a shell...
with trapped molecules inside the cage.
Chemical properties of fullerenes
Fullerene or C60 is soccer-ball-shapedTruncated icosahedron
In geometry, the truncated icosahedron is an Archimedean solid, one of thirteen convex isogonal nonprismatic solids whose faces are two or more types of regular polygons.It has 12 regular pentagonal faces, 20 regular hexagonal faces, 60 vertices and 90 edges....
or Ih with 12 pentagons and 20 hexagons. According to Euler's theorem
Euler characteristic
In mathematics, and more specifically in algebraic topology and polyhedral combinatorics, the Euler characteristic is a topological invariant, a number that describes a topological space's shape or structure regardless of the way it is bent...
these 12 pentagons are required for closure of the carbon network consisting of n hexagons and C60 is the first stable fullerene because it is the smallest possible to obey this rule. In this structure none of the pentagons make contact with each other. Both C60 and its relative C70 obey this so-called isolated pentagon rule (IPR). The next homologue C84 has 24 IPR isomers of which several are isolated and another 51,568 non-IPR isomers. Non-IPR fullerenes have thus far only been isolated as endohedral fullerenes such as Tb3N@C84 with two fused pentagons at the apex of an egg-shaped cage. or as fullerenes with exohedral stabilization such as C50Cl10 and reportedly C60H8.
Because of the molecule's spherical shape the carbon atoms are highly pyramidalized, which has far-reaching consequences for reactivity. It is estimated that strain energy
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
constitutes 80% of the heat of formation. The conjugated carbon atoms respond to deviation from planarity by orbital rehybridization of the sp² orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s and pi orbitals to a sp2.27 orbital with a gain in p-character. The p lobes extend further outside the surface than they do into the interior of the sphere and this is one of the reasons a fullerene is electronegative. The other reason is that the empty low-lying pi* orbitals also have high s character.
The double bonds in fullerene are not all the same. Two groups can be identified: 30 so-called [6,6] double bonds connect two hexagons and 60 [5,6] bonds connect a hexagon and a pentagon. Of the two the [6,6] bonds are shorter with more double-bond character and therefore a hexagon is often represented as a cyclohexatriene and a pentagon as a pentalene or [5]radialene
Radialene
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl group are also called radialenes...
. In other words, although the carbon atoms in fullerene are all conjugated the superstructure is not a super aromatic compound. The X-ray diffraction bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
values are 135.5 pm for the [6,6] bond and 146.7 pm for the [5,6] bond.
C60 fullerene has 60 pi electrons but a closed shell configuration requires 72 electrons. The fullerene is able to acquire the missing electrons by reaction with potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
to form first the salt and then the In this compound the bond length alternation observed in the parent molecule has vanished.
Fullerene reactions
Fullerenes tend to react as electrophiles. An additional driving force is relief of strainStrain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional...
when double bonds become saturated. Key in this type of reaction is the level of functionalization i.e. monoaddition or multiple additions and in case of multiple additions their topological relationships (new substituents huddled together or evenly spaced). In conformity with IUPAC rules, the terms methanofullerene are used to indicate the ring-closed (cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
) fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
derivatives, and fulleroid to ring-open (methanoannulene) structures.
Nucleophilic addition
Fullerenes react as electrophileElectrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s with a host of nucleophiles in nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
s. The intermediary formed carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
is captured by another electrophile. Examples of nucleophiles are Grignard reagents and organolithium reagent
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s. For example the reaction of C60 with methylmagnesium chloride
Methylmagnesium chloride
Methylmagnesium chloride is a commercially available Grignard reagent. Like methyllithium, it is the synthetic equivalent to the methyl carbanion synthon. It is usually sold as a solution in tetrahydrofuran. The model of the molecule shows methylmagnesium chloride with the magnesium atom in the...
stops quantitatively at the penta-adduct with the methyl groups centered around a cyclopentadienyl anion which is subsequently protonated. Another nucleophilic reaction is the Bingel reaction
Bingel reaction
The Bingel reaction in fullerene chemistry is a fullerene cyclopropanation reaction to a methanofullerene first discovered by C. Bingel in 1993 with the bromo derivative of diethyl malonate in the presence of a base such as sodium hydride or DBU...
.
Fullerene reacts with chlorobenzene
Chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.-Uses:...
and aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
in a Friedel-Crafts alkylation type reaction. In this hydroarylation the reaction product is the 1,2-addition adduct (Ar-CC-H).
Pericyclic reactions
The [6,6] bonds of fullerenes react as dienes or dienophiles in cycloadditionCycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s for instance Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s. 4-membered rings can be obtained by [2+2]cycloadditions for instance with benzyne. An example of a 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
to a 5-membered ring is the Prato reaction
Prato reaction
The Prato reaction in fullerene chemistry describes the functionalization of fullerenes and nanotubes with azomethine ylides in a 1,3-dipolar cycloaddition...
. Fullerenes react with carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s to methanofullerenes.
Hydrogenation
Fullerenes are easily hydrogenated by several methods. Examples of hydrofullerenes are C60H18 and C60H36. However, completely hydrogenated C60H60 is only hypothetical because of large strain. Highly hydrogenated fullerenes are not stable, prolonged hydrogenation of fullerenes by direct reaction with hydrogen gas at high temperature conditions results in collapse of cage structure with formation of polycyclic aromatic hydrocarbons.Oxidation
Although more difficult than reduction, oxidation of fullerene is possible for instance with oxygen and osmium tetraoxide.Hydroxylation
Fullerenes can be hydroxylated to fullerenols or fullerols. Water solubility depends on the total number of hydroxyl groups that can be attached. One method is fullerene reaction in diluted sulfuric acidSulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
and potassium nitrate
Potassium nitrate
Potassium nitrate is a chemical compound with the formula KNO3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−.It occurs as a mineral niter and is a natural solid source of nitrogen. Its common names include saltpetre , from medieval Latin sal petræ: "stone salt" or possibly "Salt...
to C60(OH)15. Another method is reaction in diluted sodium hydroxide catalysed by TBAH adding 24 to 26 hydroxyl groups. Hydroxylation has also been reported using solvent-free NaOH / hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
. C60(OH)8 was prepared using a multistep procedure starting from a mixed peroxide fullerene. The maximum number of hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups that can be attached (hydrogen peroxide method) stands at 36-40.
Electrophilic addition
Fullerenes react in electrophilic additionElectrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
s as well. The reaction with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
can add up to 24 bromine atoms to the sphere. The record holder for fluorine addition is C60F48. According to in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
predictions the as yet elusive C60F60 may have some of the fluorine atoms in endo positions (pointing inwards) and may resemble a tube more than it does a sphere.
Retro additions
Protocols have been investigated for removing substituents via retroadditions after they have served their purpose. Examples are the retro-Bingel reaction and the retro-Prato reaction.Fullerenes as ligands
Fullerene is a ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
. The [6,6] double bond is electron-deficient and usually forms metallic bonds with η = 2 hapticity
Hapticity
The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to...
. Bonding modes such as η = 5 or η = 6 can be induced by modification of the coordination sphere
Coordination sphere
In coordination chemistry, the coordination sphere refers to a central atom or ion and an array of molecules or anions, the ligands, around.Molecules that are attached noncovalently to the ligands are called the second coordination sphere....
.
- C60 fullerene reacts with tungsten hexacarbonylTungsten hexacarbonylTungsten hexacarbonyl is the chemical compound with the formula W6. This complex gave rise to the first example of a dihydrogen complex....
W(CO)6 to the (η²-C60)W(CO)5 complex in a hexaneHexaneHexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
solution in direct sunlight.
Multistep fullerene synthesis
Although the procedure for the synthesis of the C60 fullerene is well established (generation of a large current between two nearby graphite electrodes in an inert atmosphere) a 2002 study described an organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of the compound starting from simple organic compounds.
In the final step a large polycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbons , also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are potent atmospheric pollutants that consist of fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH...
consisting of 13 hexagons and three pentagons is submitted to flash vacuum pyrolysis at 1100°C and 0.01 Torr
Torr
The torr is a non-SI unit of pressure with the ratio of 760 to 1 standard atmosphere, chosen to be roughly equal to the fluid pressure exerted by a millimetre of mercury, i.e., a pressure of 1 torr is approximately equal to 1 mmHg...
. The three carbon chlorine bonds serve as free radical incubators and the ball is stitched up in a no-doubt complex series of radical reactions. The chemical yield is low: 0.1 to 1%. A small percentage of fullerenes is formed in any process which involves burning of hydrocarbons, e.g. in candle burning. The yield through a combustion method is often above 1%. The method proposed above does not provide any advantage for synthesis of fullerenes compared to the usual combustion method, and therefore, the organic synthesis of fullerenes remains a challenge for chemistry.
A similar exercise aimed at construction of a C78 cage in 2008 (but leaving out the precursor's halogens) did not result in a sufficient yield but at least the introduction of Stone Wales defect
Stone Wales defect
A Stone–Wales defect is a crystallographic defect that occurs on carbon nanotubes and graphene and is thought to have important implications for nanotube's mechanical properties. The defect is named after Anthony Stone and David Wales of Cambridge University, who described it in a 1986 paper on the...
s could be ruled out.
Multistep nanoribbon synthesis
In the field of graphene nanoribbonsGraphene nanoribbons
Graphene nanoribbons , often abbreviated GNRs, are thin strips of graphene or unrolled single-walled carbon nanotubes...
a bottom-up approach has also been investigated
Open-cage fullerenes
A part of fullerene research is devoted to so-called open-cage fullerenes whereby one or more bonds are removed chemically exposing an orifice. In this way it is possible to insert into it small molecules such as hydrogen, helium or lithium. The first such open-cage fullerene was reported in 1995. In endohedral hydrogen fullereneEndohedral hydrogen fullerene
Endohedral hydrogen fullerene or H2@C60 is an endohedral fullerene containing molecular hydrogen. This chemical compound has a potential application in molecular electronics and was synthesized in 2005 at Kyoto University by the group of Koichi Komatsu...
s the opening, hydrogen insertion and closing back up has already been demonstrated.
Fullerene dimers
The C60 fullerene dimerizes in a formal [2+2] cycloadditionCycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
to a C120 bucky dumbbell in the solid state by mechanochemistry
Mechanochemistry
Mechanochemistry is the coupling of the mechanical and the chemical phenomena on a molecular scale and includes mechanical breakage, chemical behaviour of mechanically-stressed solids , tribology, polymer degradation under shear, cavitation-related phenomena , shock wave chemistry and physics, and...
(high-speed vibration milling) with potassium cyanide
Potassium cyanide
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and...
as a catalyst. The trimer has also been reported using 4-aminopyridine
4-Aminopyridine
4-Aminopyridine is an organic compound with the chemical formula C5H4N–NH2. The molecule is one of the three isomeric amines of pyridine...
as catalyst (4% yield) and observed with Scanning Tunneling Microscopy as a monolayer
Monolayer
- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
Nanotube chemistry
Carbon nanotubeCarbon nanotube
Carbon nanotubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1, significantly larger than for any other material...
s, also part of the fullerene family, can be described as graphene
Graphene
Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer...
sheets rolled into a cylindrical tube. Unlike the spherical fullerenes made up of hexagons and pentagons, nanotubes only have hexagons present but in terms of reactivity both systems have much in common. Due to electrostatic forces nanotubes have a nasty tendency to cluster together into bundles and many potential applications require an exfoliation process. One way to do this is by chemical surface modification.
A useful tool for the analysis of derivatised nanotubes is Raman spectroscopy
Raman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
which shows a G-band (G for graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
) for the native nanotubes at 1580 cm−1 and a D-band (D for defect) at 1280 cm−1 when the graphite lattice is disrupted with conversion of sp² to sp³ hybridized carbon. The ratio of both peaks ID/IG is taken as a measure of functionalization. Other tools are UV spectroscopy where pristine nanotubes show distinct Van Hove singularities
Van Hove singularity
A Van Hove singularity is a kink in the density of states of a solid. The wavevectors at which Van Hove singularities occur are often referred to as critical points of the Brillouin zone...
where functionalized tubes do not, and simple TGA analysis
Thermogravimetric analysis
Thermogravimetric analysis or thermal gravimetric analysis is a type of testing performed on samples that determines changes in weight in relation to change in temperature. Such analysis relies on a high degree of precision in three measurements: weight, temperature, and temperature change...
.
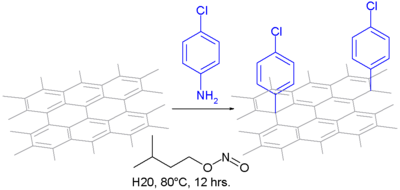
In one type of chemical modification, aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
is oxidized to a diazonium intermediate. After expulsion of nitrogen, it forms a covalent bond as an aryl radical
Aryl radical
An Aryl radical in organic chemistry is an reactive intermediate and an arene compound incorporating one free radical carbon atom as part of the ring structure. As such it is the radical counterpart of the Arenium ion. The parent compound is the phenyl radical C6H5....
:

Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s , 1,3-dipolar cycloadditions of
azomethine ylides and azide–alkyne cycloaddition reactions. One example is a DA reaction assisted by chromium hexacarbonyl and high pressure. The ID/IG ratio for reaction with Danishefsky’s diene
Danishefsky’s diene
Danishefsky’s diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions...
is 2.6.
Nanotubes can also be alkylated with alkyl halides using lithium or sodium metal and liquid ammonia (Birch reduction
Birch reduction
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
conditions). The initial nanotube salt can function as an polymerization initiator and can react with peroxides to form alkoxy functionalized nanotubes
Fullerene purification
Fullerene purification is the process of obtaining a fullereneFullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
compound free of contamination. In fullerene production mixtures of C60, C70 and higher homologues are always formed. Fullerene purification is key to fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
science and determines fullerene prices and the success of practical applications of fullerenes. The first available purification method for C60 fullerene was by HPLC
High-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
from which small amounts could be generated at large expense.
A practical laboratory-scale method for purification of soot enriched in C60 and C70 starts with extraction in toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
followed by filtration
Filtration
Filtration is commonly the mechanical or physical operation which is used for the separation of solids from fluids by interposing a medium through which only the fluid can pass...
with a paper filter. The solvent is evaporated and the residue (the toluene-soluble soot fraction) redissolved in toluene and subjected to column chromatography
Column chromatography
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability...
. C60 elutes first with a purple color and C70 is next displaying a reddish-brown color.
In nanotube processing the established purification method for removing amorphous carbon and metals is by competitive oxidation (often a sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
/ nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
mixture). It is assumed that this oxidation creates oxygen containing groups (hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
, carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
, carboxyl) on the nanotube surface which electrostatically stabilize them in water and which can later be utilized in chemical functionalization. One report reveals that the oxygen containing groups in actuality combine with carbon contaminations absorbed to the nanotube wall that can be removed by a simple base wash. Cleaned nanotubes are reported to have reduced D/G ratio indicative of less functionalization, and the absence of oxygen is also apparent from IR spectroscopy and X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
.
Experimental purification strategies
A recent kilogram-scale fullerene purification strategy was demonstrated by Nagata et al. In this method C60 was separated from a mixture of C60, C70 and higher fullerene compounds by first adding the amidineAmidine
Amidines are a class of oxoacid derivatives.The oxoacid from which an amidine is derived must be of the form RnEOH, where R is a substituent...
compound DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
to a solution of the mixture in 1,2,3-trimethylbenzene
1,2,3-Trimethylbenzene
1,2,3-Trimethylbenzene is a benzene derivative. It is one of the three isomers of trimethylbenzene.-References:...
. DBU as it turns out only reacts to C70 fullerenes and higher which reaction products separate out and can be removed by filtration. C60 fullerenes do not have any affinity for DBU and are subsequently isolated. Other diamine compounds like DABCO
DABCO
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base. It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the...
do not share this selectivity.
C60 but not C70 forms a 1:2 inclusion compound
Inclusion compound
In host-guest chemistry an inclusion compound is a complex in which one chemical compound forms a cavity in which molecules of a second "guest" compound are located. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which...
with cyclodextrin
Cyclodextrin
Cyclodextrins are a family of compounds made up of sugar molecules bound together in a ring ....
(CD). A separation method for both fullerenes based on this principle is made possible by anchoring cyclodextrin to colloidal gold
Colloidal gold
Colloidal gold is a suspension of sub-micrometre-sized particles of gold in a fluid — usually water. The liquid is usually either an intense red colour , or a dirty yellowish colour ....
particles through a sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
-sulfur bridge. The Au/CD compound is very stable and soluble in water and selectively extracts C60 from the insoluble mixture after reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
ing for several days. The C70 fullerene component is then removed by simple filtration
Filtration
Filtration is commonly the mechanical or physical operation which is used for the separation of solids from fluids by interposing a medium through which only the fluid can pass...
. C60 is driven out from the Au/CD compound by adding adamantol
Adamantane
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
which has a higher affinity for the cyclodextrin cavity. Au/CD is completely recycled
Recycling
Recycling is processing used materials into new products to prevent waste of potentially useful materials, reduce the consumption of fresh raw materials, reduce energy usage, reduce air pollution and water pollution by reducing the need for "conventional" waste disposal, and lower greenhouse...
when adamantol in turn is driven out by adding ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and ethanol removed by evaporation. 50 mg of Au/CD captures 5 mg of C60 fullerene.