Carbene
Encyclopedia

Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a carbene is a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
containing a neutral carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom with a valence
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
of two and two unshared valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
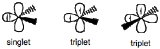
, the parent hydride to which all other carbene compounds are related. Carbenes are classified as either singlets or triplets
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
depending upon their electronic structure. Most carbenes are very short lived, although persistent carbene
Persistent carbene
A persistent carbene is a type of carbene demonstrating particular stability. The best-known examples are diaminocarbenes with the general formula 2C:, where the 'R's are various functional groups...
s are known.
One well studied carbene is Cl2C:, or dichlorocarbene
Dichlorocarbene
Dichlorocarbene is a carbene commonly encountered in organic chemistry. This reactive intermediate with chemical formula CCl2 is easily available by reaction of chloroform and a base such as potassium t-butoxide or sodium hydroxide dissolved in water...
, which can be generated in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
from chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
and a strong base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
.
Structure and bonding

Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
and triplet
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
carbenes. Singlet carbenes are spin-paired. In the language of valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
, the molecule adopts an sp2 hybrid structure
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
. Triplet carbenes have two unpaired electrons. They may be either linear or bent, i.e. sp or sp2 hybridized, respectively. Most carbenes have a nonlinear triplet ground state, except for those with nitrogen, oxygen, or sulfur atoms, and halides directly bonded to the divalent carbon.
Carbenes are called singlet or triplet depending on the electronic spins
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
they possess. Triplet carbenes are paramagnetic and may be observed by electron spin resonance spectroscopy if they persist long enough. The total spin of singlet carbenes is zero while that of triplet carbenes is one (in units of
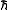 ). Bond angles are 125-140° for triplet methylene
). Bond angles are 125-140° for triplet methyleneMethylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
and 102° for singlet methylene (as determined by EPR
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
). Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media.
For simple hydrocarbons, triplet carbenes usually have energies 8 kcal/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
(33 kJ/mol) lower than singlet carbenes (see also Hund's rule of Maximum Multiplicity
Hund's rule of Maximum Multiplicity
Hund's Rule of Maximum Multiplicity is an observational rule which states that a greater total spin state usually makes the resulting atom more stable. Accordingly, it can be taken that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in...
), thus, in general, triplet is the more stable state (the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
) and singlet is the excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
species. Substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s that can donate electron pair
Electron pair
In chemistry, an electron pair consists of two electrons that occupy the same orbital but have opposite spins.Because electrons are fermions, the Pauli exclusion principle forbids these particles from having exactly the same quantum numbers. Therefore the only way to occupy the same orbital, i.e....
s may stabilize the singlet state by delocalizing the pair into an empty p-orbital. If the energy of the singlet state is sufficiently reduced it will actually become the ground state.
No viable strategies exist for triplet stabilization. The carbene called 9-fluorenylidene has been shown to be a rapidly equilibrating
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
mixture of singlet and triplet states with an approximately 1.1 kcal/mol (4.6 kJ/mol) energy difference. It is, however, debatable whether diaryl carbenes such as the fluorene
Fluorene
Fluorene, or 9H-fluorene, is a polycyclic aromatic hydrocarbon. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It is combustible. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar...
carbene are true carbenes because the electrons can delocalize to such an extent that they become in fact biradicals. In silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
experiments suggest that triplet carbenes can be stabilized with electropositive groups such as trifluorosilyl groups.
Reactivity

Cheletropic reaction
thumb|300px|right|Pericyclic ReactionsCheletropic reactions are a type of pericyclic reaction. A pericyclic reaction is one that involves a transition state with a cyclic array of atoms and an associated cyclic array of interacting orbitals. A reorganization of σ and π bonds occurs in this cyclic...
s as either electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s or nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s. Singlet carbenes with unfilled p-orbital should be electrophilic. Triplet carbenes can be considered to be diradicals, and participate in stepwise radical additions. Triplet carbenes have to go through an intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
with two unpaired electrons whereas singlet carbene can react in a single concerted
Concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not to depend on solvent polarity ruling out large buildup...
step.
Due to these two modes of reactivity, reactions of singlet methylene are stereospecific whereas those of triplet methylene are stereoselective. This difference can be used to probe the nature of a carbene. For example, the reaction of methylene generated from photolysis of diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
with cis-2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
or with trans-2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
each give a single diastereomer of the 1,2-dimethylcyclopropane product: cis from cis and trans from trans, which proves that the methylene is a singlet. If the methylene were a triplet, one would not expect the product to depend upon the starting alkene geometry, but rather a nearly identical mixture in each case.
Reactivity of a particular carbene depends on the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
groups. Their reactivity can be affected by metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
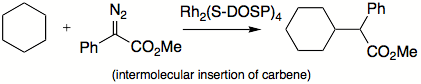
s. Some of the reactions carbenes can do are insertions into C-H bonds
Carbene C-H insertion
Carbene C−H insertion in organic chemistry concerns the insertion reaction of a carbene into a carbon–hydrogen bond. This organic reaction is of some importance in the synthesis of new organic compounds . Simple carbenes such as methylene and dichlorocarbene are not regioselective towards...
, skeletal rearrangements, and additions to double bonds. Carbenes can be classified as nucleophilic, electrophilic, or ambiphilic. For example, if a substituent is able to donate a pair of electrons, most likely carbene will not be electrophilic. Alkyl carbenes insert much more selectively than methylene, which does not differentiate between primary, secondary, and tertiary C-H bonds.
Cyclopropanation
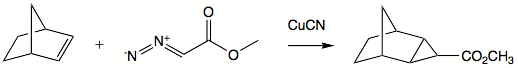
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
in the product molecule. Addition reactions are commonly very fast and exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
. The slow step in most instances is generation of carbene. A well-known reagent employed for alkene-to-cyclopropane reactions is Simmons-Smith reagent. This reagent is a system of copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, where the active reagent is believed to be iodomethylzinc iodide
Iodomethylzinc iodide
Iodomethylzinc iodide is the active reagent in the Simmons-Smith reaction. For example, iodomethylzinc iodide, formed in situ from diiodomethane and a zinc-copper couple reacts with cyclohexene to give norcarane ....
. Reagent is complexed by hydroxy
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups such that addition commonly happens syn
SYN
Syn may refer to:*SYN , a band by HALCA *Doctor Syn, a character in novels by Russell Thorndike*Grand Admiral Peccati Syn character in the Star Wars expanded universe*Syn , in Norse mythology...
to such group.
C—H insertion
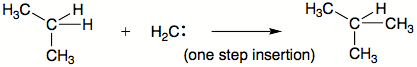
Carbene C-H insertion
Carbene C−H insertion in organic chemistry concerns the insertion reaction of a carbene into a carbon–hydrogen bond. This organic reaction is of some importance in the synthesis of new organic compounds . Simple carbenes such as methylene and dichlorocarbene are not regioselective towards...
are another common type of carbene reactions. The carbene basically interposes itself into an existing bond. The order of preference is commonly: 1. X–H bonds where X is not carbon 2. C–H bond 3. C–C bond. Insertions may or may not occur in single step.
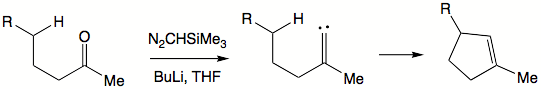
Intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
insertion reactions present new synthetic solutions. Generally, rigid structures favor such insertions to happen. When an intramolecular insertion is possible, no intermolecular insertions are seen. In flexible structures, five-membered ring formation is preferred to six-membered ring formation. Both inter- and intramolecular insertions are amendable to asymmetric induction by choosing chiral ligands on metal centers.
Alkylidene carbenes are alluring in that they offer formation of cyclopentene
Cyclopentene
Cyclopentene is a chemical compound with the formula 58. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes.Cyclopentene is produced industrially in large amounts...
moieties. To generate an alkylidene carbene a ketone can be exposed to trimethylsilyl
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
.
Carbenes ligands in organometallic chemistry
In organometallicOrganometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
species, metal complexes with the formulae LnMCRR' are often described as carbene complexes. Such species do not however react like free carbenes and are rarely generated from carbene precursors, except for the persistent carbenes. The transition metal carbene complex
Transition metal carbene complex
A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and...
es can be classified according to their reactivity, with the first two classes being the most clearly defined:
- Fischer carbenes, in which the carbene is bonded to a metal that bears an electron-withdrawing group (usually a carbonyl). In such cases the carbenoid carbon is mildly electrophilic.
- Schrock carbenes, in which the carbene is bonded to a metal that bears an electron-donating groups. In such cases the carbenoid carbon is nucleophilic and resembles Wittig reagent (which are not considered carbene derivatives).
- Persistent carbenePersistent carbeneA persistent carbene is a type of carbene demonstrating particular stability. The best-known examples are diaminocarbenes with the general formula 2C:, where the 'R's are various functional groups...
s, also known as ArduengoAnthony Joseph Arduengo IIIAnthony Joseph Arduengo, III is the Saxon Professor of Chemistry at the University of Alabama and an adjunct professor at the Institute for Inorganic Chemistry of Braunschweig University of Technology in Germany...
or WanzlickWanzlick equilibriumThe Wanzlick equilibrium is a chemical equilibrium between a relatively stable carbene compound and its dimer.-Original conjecture:In 1960, H.-W. Wanzlick and E...
carbenes. These include the class of N-heterocyclic carbenes (NHCs) and are often are used as ancillary ligandLigandIn coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s in organometallic chemistry. Such carbenes are spectator ligands of low reactivity.
Generation of carbenes
- A method that is broadly applicable to organic synthesis is induced elimination of halides from gem-dihalides employing organolithium reagentOrganolithium reagentAn organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s. It remains uncertain if under these conditions free carbenes are formed or metal-carbene complex. Nevertheless, these metallocarbenes (or carbenoids) give the expected organic products.
- R2CBr2 + BuLi → R2CLi(Br) + BuBr
- R2CLi(Br) → R2C + LiBr
For cyclopropanations, zinc is employed in the Simmons–Smith reaction. In a specialized but instructive case, alpha-halomercury compounds can be isolated and separately thermolyzed. For example, the "Seyferth reagent" C6H5HgCCl3 releases CCl2 upon heating, concomitant with formation of C6H5HgCl.
- Most commonly, carbenes are generated from diazoalkanes, via photolytic, thermal, or transition metalTransition metalThe term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
-catalyzed routes. Catalysts typically feature rhodiumRhodiumRhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
and copperCopperCopper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
. The Bamford-Stevens reactionBamford-Stevens reactionThe Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens...
gives carbenes in aprotic solvents and carbenium ions in protic solvents. - Base-induced elimination HX from haloforms (CHX3) with under phase-transfer conditions.
- Photolysis of diazirines and epoxideEpoxideAn epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s can also be employed. Diazirines are cyclic forms of diazoalkanes. The strain of the small ring makes photoexcitation easy. Photolysis of epoxides gives carbonylCarbonylIn organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds as side products. With asymmetric epoxides, two different carbonyl compounds can potentially form. The nature of substituents usually favors formation of one over the other. One of the C-O bonds will have a greater double bond character and thus will be stronger and less likely to break. Resonance structures can be drawn to determine which part will contribute more to the formation of carbonyl. When one substituent is alkyl and another arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
, the aryl-substituted carbon is usually released as a carbene fragment. - Carbenes are intermediates in the Wolff rearrangementWolff rearrangementThe Wolff rearrangement is a rearrangement reaction converting a α-diazo-ketone into a ketene. This reaction was first reported by Ludwig Wolff in 1912....
Applications of carbenes
A large scale application of carbenes is the industrial production of tetrafluoroethyleneTetrafluoroethylene
Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:...
, the precursor to Teflon. Tetrafluoroethylene is generated via the intermediacy of difluorocarbene
Difluorocarbene
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 msec, in solution and in the gas phase, respectively...
:
- CHClF2 → CF2 + HCl
- 2 CF2 → F2C=CF2
History
Carbenes have first been postulated by Eduard BuchnerEduard Buchner
Eduard Buchner was a German chemist and zymologist, awarded with the 1907 Nobel Prize in Chemistry thanks to his work on fermentation.-Early years:...
in 1903 in cyclopropanation studies of ethyl diazoacetate
Ethyl diazoacetate
Ethyl diazoacetate is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetate in water....
with toluene. In 1912 Hermann Staudinger
Hermann Staudinger
- External links :* Staudinger's * Staudinger's Nobel Lecture *....
also converted alkenes to cyclopropanes with diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
and CH2 as an intermediate. Doering
William von Eggers Doering
William von Eggers Doering was a Professor Emeritus at Harvard University and the former Chair of its Chemistry Department...
in 1954 demonstrated with dichlorocarbene
Dichlorocarbene
Dichlorocarbene is a carbene commonly encountered in organic chemistry. This reactive intermediate with chemical formula CCl2 is easily available by reaction of chloroform and a base such as potassium t-butoxide or sodium hydroxide dissolved in water...
synthetic utility.
See also
- Transition metal carbene complexTransition metal carbene complexA transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and...
also known as carbenoids - Atomic carbonAtomic carbonAtomic carbon in chemistry is single carbon atom with chemical formula :C: - in effect a dicarbene.This very short lived species is created by passing a large current through two adjacent carbon rods, generating an electric arc. Atomic carbon is generated in the process...
a single carbon atom with chemical formula :C:, in effect a dicarbene. Also has been used to make "true carbenes" in situ. - Foiled carbeneFoiled carbeneA foiled carbene in organic chemistry is a special type of stabilized carbene due to the proximity of a double bond. This type of reactive intermediate is implicated in certain organic reactions...
s derive their stability from proximity of a double bond (i.e. their ability to form conjugated systems). - Carbene analogs