
Endohedral fullerenes
Encyclopedia
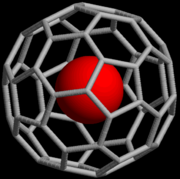
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
s that have additional atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s, ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s, or cluster
Cluster (physics)
In physics, the term clusters denotes small, multiatom particles. As a rule of thumb, any particle of somewhere between 3 and 3x107 atoms is considered a cluster. Two-atom particles are sometimes considered clusters as well....
s enclosed within their inner spheres. The first lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
C60 complex was synthesized
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
in 1985 called La@C60. The @ sign in the name reflects the notion of a small molecule trapped inside a shell. Two types of endohedral complexes exist: endohedral metallofullerenes and non-metal doped fullerenes .
Endohedral metallofullerenes
Doping fullerenes with electropositive metals takes place in an arc reactorArc reactor
Arc reactor may refer to:* an apparatus for producing C60 and other fullerenes, with or without metal doping* Plasma arc waste disposal reactor, a kind of waste processing system...
or via laser evaporation. The metals can be transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s like scandium
Scandium
Scandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic transition metal, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids...
, yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
as well as lanthanides like lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
and cerium
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
. Also possible are endohedral complexes with elements of the alkaline earth metal
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
s like barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
and strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
, alkali metal
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
s like potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
and tetravalent metals like uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
, zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
and hafnium
Hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable...
. The synthesis in the arc reactor is however unspecific. Besides unfilled fullerenes, endohedral metallofullerenes develop with different cage sizes like La@C60 or La@C82 and as different isomer cages. Aside from the dominant presence of mono-metal cages, numerous di-metal endohedral complexes and the tri-metal carbide fullerenes like Sc3C2@C80 were also isolated.
In 1998 a discovery drew large attention. With the synthesis of the Sc3N@C80, the inclusion of a molecule fragment in a fullerene cage had succeeded for the first time. This compound can be prepared by arc-vaporization at temperatures up to 1100 °C of graphite rods packed with scandium(III) oxide
Scandium(III) oxide
Scandium oxide, Sc2O3, scandia is a high melting white solid used in high-temperature systems , electronic ceramics, and glass composition .-Physical and chemical properties:...
iron nitride
Iron nitride
- Chemical properties:Iron has four nitrides, Fe2N, Fe3N1+x, Fe4N, and Fe16N2. They are crystalline, metallic solids. Group VII and group VIII transition metals form nitrides that decompose at relatively low temperatures - iron nitride, Fe2N decomposes under loss of molecular nitrogen at about...
and graphite powder in a K-H generator in a nitrogen atmosphere at 300 Torr
Torr
The torr is a non-SI unit of pressure with the ratio of 760 to 1 standard atmosphere, chosen to be roughly equal to the fluid pressure exerted by a millimetre of mercury, i.e., a pressure of 1 torr is approximately equal to 1 mmHg...
.
Endohedral metallofullerenes are characterised by the fact that electrons will transfer from the metal atom to the fullerene cage and that the metal atom takes a position off-center in the cage. The size of the charge transfer
Charge transfer complex
A charge-transfer complex or electron-donor-acceptor complex is an association of two or more molecules, or of different parts of one very large molecule, in which a fraction of electronic charge is transferred between the molecular entities. The resulting electrostatic attraction provides a...
is not always simple to determine. In most cases it is between 2 and 3 charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
units, in the case of the La2@C80 however it can be even about 6 electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s such as in Sc3N@C80 which is better described as [Sc3N]+6@[C80]-6. These anionic fullerene cages are very stable molecules and do not have the reactivity associated with ordinary empty fullerenes. They are stable in air up to very high temperatures (600 to 850°C) and the Prato reaction
Prato reaction
The Prato reaction in fullerene chemistry describes the functionalization of fullerenes and nanotubes with azomethine ylides in a 1,3-dipolar cycloaddition...
yields only a monoadduct and not multi-adducts as with empty fullerenes.
The lack of reactivity in Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s is utilised in a method to purify [C80]-6 compounds from a complex mixture of empty and partly filled fullerenes of different cage size . In this method Merrifield resin
Merrifield resin
Merrifield Resin is a polystyrene resin based on a copolymer of styrene and chloromethylstyrene. In addition this polymer is also cross-linked with divinylbenzene present in the monomer composition up to 5%. Merrifield resin is named after its inventor, Robert Bruce Merrifield , and used in...
is modified as a cyclopentadienyl
Cyclopentadienyl complex
A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic...
resin and used as a solid phase against a mobile phase containing the complex mixture in a column chromatography
Column chromatography
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability...
operation. Only very stable fullerenes such as [Sc3N]+6@[C80]-6 pass through the column unreacted.
In Ce2@C80 the two metal atoms exhibit a non-bonded interaction. Since all the six-membered rings in C80-Ih are equal the two encapsulated Ce atoms exhibit a three-dimensional random motion . This is evidenced by the presence of only two signals in the 13C-NMR
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
spectrum. It is possible to force the metal atoms to a standstill at the equator as shown by x-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
when the fullerene is exahedrally functionalized by an electron donation silyl
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
group in a reaction of Ce2@C80 with 1,1,2,2-tetrakis(2,4,6-trimethylphenyl)-1,2-disilirane.
Non-metal doped fullerenes
Saunders in 1993 showed the formation of endohedral complexes He@C60 and Ne@C60 when C60 is exposed to a pressure of around 3 barBar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
of the noble gases. Under these conditions about one out of every 650,000 C60 cages was doped with a helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
atom.
The formation of endohedral complexes with helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
, argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
, krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
and xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
as well as numerous adducts of the He@C60 compound was also demonstrated with pressures of 3 kbars and incorporation of up to 0.1% of the noble gases.
While noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es are chemically very inert and commonly exist as individual atoms, this is not the case for nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and so the formation of the endohedral complexes N@C60, N@C70 and P@C60 is more surprising.
The nitrogen atom is in its electronic initial state (4S3/2) and is therefore to be highly reactive. Nevertheless N@C60 is sufficiently stable that exohedral derivatization from the mono- to the hexa adduct of the malonic acid
Malonic acid
Malonic acid is a dicarboxylic acid with structure CH22. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester...
ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
is possible.
In these compounds no charge transfer of the nitrogen atom in the center to the carbon atoms of the cage takes place. Therefore 13C-couplings
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, which are observed very easily with the endohedral metallofullerenes, could only be observed in the case of the N@C60 in a high resolution spectrum as shoulders of the central line.
The central atom in these endohedral complexes is located in the center of the cage. While other atomic traps require complex equipment, e.g. laser cooling
Laser cooling
Laser cooling refers to the number of techniques in which atomic and molecular samples are cooled through the interaction with one or more laser light fields...
or magnetic trap
Magnetic trap
Magnetic trap refers to one of three types of traps used for atoms or charged particles:* Magnetic trap , used to trap neutral atoms in a magnetic field gradient...
s, endohedral fullerenes represent an atomic trap that is stable at room temperature and for an arbitrarily long time. Atomic or ion traps are of great interest since particles are present free from (significant) interaction with their environment, allowing unique quantum mechanical phenomena to be explored. For example, the compression of the atomic wave function as a consequence of the packing in the cage could be observed with ENDOR spectroscopy. The nitrogen atom can be used as a probe, in order to detect the smallest changes of the electronic structure of its environment.
Contrary to the metallo endohedral compounds, these complexes cannot be produced in an arc. Atoms are implanted in the fullerene starting material using gas discharge (nitrogen and phosphorus complexes) or by direct ion implantation
Ion implantation
Ion implantation is a materials engineering process by which ions of a material are accelerated in an electrical field and impacted into another solid. This process is used to change the physical, chemical, or electrical properties of the solid...
. Alternatively, endohedral hydrogen fullerene
Endohedral hydrogen fullerene
Endohedral hydrogen fullerene or H2@C60 is an endohedral fullerene containing molecular hydrogen. This chemical compound has a potential application in molecular electronics and was synthesized in 2005 at Kyoto University by the group of Koichi Komatsu...
s can be produced by opening and closing a fullerene by organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
methods.

