
Butyl
Encyclopedia
In organic chemistry
, butyl is a four-carbon
alkyl radical
or substituent group with general chemical formula
-C4H9, derived from either of the two isomer
s of butane
.
The isomer n-butane can connect either at one of the two terminal carbon
atoms or at one of the two internal carbon atoms, giving rise to two "-butyl" groups:
The second, branched isomer of butane, isobutyl, can connect either at one of the three terminal carbons or at the central carbon, giving rise to another two groups:
, "isobutyl", "sec-butyl", and "tert-butyl" are all retained trivial names.
Butyl is the largest substituent for which trivial name
s are commonly used for all isomers.
The butyl group's carbon that is connected to the rest (R) of the molecule is called the RI or R-prime carbon . The prefixes sec (from "secondary") and tert (from "tertiary") refer to the number of additional side chain
s connected to the first butyl carbon. The prefix "iso" (from "isomer") means "equal" while the prefix' n-' stands for "normal".
numbers. The name is derived from butyric acid
, a four-carbon carboxylic acid
found in rancid
butter
. The name of butyric acid, in turn, comes from Latin
butyrum, butter.
is very bulky and used in chemistry for kinetic stabilisation together with other bulky groups such as the related trimethylsilyl
group. The effect that the t-butyl group exerts on the progress of a chemical reaction is called the tert-butyl effect.
This effect is illustrated in the Diels-Alder reaction
below, where the tert-butyl substituent causes a reaction rate
acceleration by a factor of 240 compared to hydrogen as the substituent.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, butyl is a four-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
alkyl radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
or substituent group with general chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
-C4H9, derived from either of the two isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s of butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
.
The isomer n-butane can connect either at one of the two terminal carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms or at one of the two internal carbon atoms, giving rise to two "-butyl" groups:
- Normal butyl or n-Butyl: CH3–CH2–CH2–CH2– (fully systematic name: butyl)
- Secondary butyl or sec-Butyl: CH3–CH2–CH(CH3)– (fully systematic name: 1-methylpropyl)
The second, branched isomer of butane, isobutyl, can connect either at one of the three terminal carbons or at the central carbon, giving rise to another two groups:
- Isobutyl: (CH3)2CH–CH2– (fully systematic name: 2-methylpropyl)
- Tertiary butyl, tert-Butyl or t-butyl: (CH3)3C– (fully systematic name: 1,1-dimethylethyl)
Nomenclature
According to IUPAC nomenclatureIUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
, "isobutyl", "sec-butyl", and "tert-butyl" are all retained trivial names.
| Skeletal formula Skeletal formula The skeletal formula of an organic compound is a shorthand representation of its molecular structure, developed by the organic chemist, Friedrich August Kekulé von Stradonitz. Skeletal formulae are ubiquitous in organic chemistry, because they are relatively quick and simple to draw. Carbon and... |
Common name Common name A common name of a taxon or organism is a name in general use within a community; it is often contrasted with the scientific name for the same organism... |
IUPAC name | Systematic name Systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection... |
Alternate notation |
|---|---|---|---|---|
| n-butyl | butyl | butyl | butan-1-yl | |
| isobutyl | isobutyl | 2-methylpropyl | 2-methylpropan-1-yl | |
| sec-butyl | sec-butyl | 1-methylpropyl | butan-2-yl | |
| tert-butyl | tert-butyl | 1,1-dimethylethyl | 2-methylpropan-2-yl |
Butyl is the largest substituent for which trivial name
Trivial name
In chemistry, a trivial name is a common name or vernacular name; it is a non-systematic name or non-scientific name. That is, the name is not recognised according to the rules of any formal system of nomenclature...
s are commonly used for all isomers.
The butyl group's carbon that is connected to the rest (R) of the molecule is called the RI or R-prime carbon . The prefixes sec (from "secondary") and tert (from "tertiary") refer to the number of additional side chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
s connected to the first butyl carbon. The prefix "iso" (from "isomer") means "equal" while the prefix
Some examples
The following are the four isomers of "butyl acetate":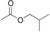 |
|||
Butyl acetate n-Butyl acetate, also known as butyl ethanoate, is an organic compound commonly used as a solvent in the production of lacquers and other products. It is also used as a synthetic fruit flavoring in foods such as candy, ice cream, cheeses, and baked goods. Butyl acetate is found in many types of... |
Isobutyl acetate The chemical compound isobutyl acetate, also known as 2-methylpropyl ethanoate or β-methylpropyl acetate, is a common solvent. It is produced from the esterification of isobutanol with acetic acid. It is used as a solvent for lacquer and nitrocellulose... |
Sec-Butyl acetate sec-Butyl acetate, or s-butyl acetate, is a solvent commonly used as a solvent in lacquers and enamels, where it is used in the production of acyclic polymers, vinyl resins, and nitrocellulose... |
Tert-Butyl acetate tert-Butyl acetate, or t-butyl acetate is a colourless flammable liquid with a camphor- or blueberry-like smell. It is used as a solvent in the production of lacquers, enamels, inks, adhesives, thinners and industrial cleaners... |
Etymology
As the number of carbons in an alkyl chain increases, butyl is the last to be named historically instead of through GreekGreek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
numbers. The name is derived from butyric acid
Butyric acid
Butyric acid , also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates...
, a four-carbon carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
found in rancid
Rancidification
Rancidification is the chemical decomposition of fats, oils and other lipids . When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable . In processed meats, these flavors are collectively known as "warmed over flavor"...
butter
Butter
Butter is a dairy product made by churning fresh or fermented cream or milk. It is generally used as a spread and a condiment, as well as in cooking applications, such as baking, sauce making, and pan frying...
. The name of butyric acid, in turn, comes from Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
butyrum, butter.
Tert-butyl effect
The tert-butyl substituentSubstituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
is very bulky and used in chemistry for kinetic stabilisation together with other bulky groups such as the related trimethylsilyl
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
group. The effect that the t-butyl group exerts on the progress of a chemical reaction is called the tert-butyl effect.
This effect is illustrated in the Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
below, where the tert-butyl substituent causes a reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
acceleration by a factor of 240 compared to hydrogen as the substituent.

