
Magnetochemistry
Encyclopedia
Magnetochemistry is concerned with the magnetic properties of chemical compound
s. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons
. Molecular compounds that contain one or more unpaired electron
s are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metal
s the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanide
s and actinide
s, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism
, antiferromagnetism
or ferrimagnetism
depending on the relative orientations of the individual spins.
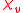 is defined by the relationship
is defined by the relationship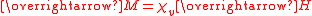
where, is the magnetization
is the magnetization
of the material (the magnetic dipole moment per unit volume), measured in ampere
s per meter ( SI
units), and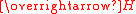 is the magnetic field strength, also measured in amperes per meter. Susceptibility is a dimensionless quantity
is the magnetic field strength, also measured in amperes per meter. Susceptibility is a dimensionless quantity
. For chemical applications the molar magnetic susceptibility (χmol) is the preferred quantity. It is measured in m3·mol−1 (SI) or cm3·mol−1 (CGS) and is defined as
where ρ is the density
in kg·m−3 (SI) or g·cm−3 (CGS) and M is molar mass
in kg·mol−1 (SI) or g·mol−1 (CGS).
A variety of methods are available for the measurement of magnetic susceptibility.
there is an interaction because each electron
in the atom behaves like a magnet, that is, the electron has a magnetic moment
. There are two type of interaction.
When the atom is present in a chemical compound
its magnetic behaviour is modified by its chemical environment. Measurement of the magnetic moment can give useful chemical information.
In certain crystalline materials individual magnetic moments may be aligned with each other (magnetic moment has both magnitude and direction). This gives rise to ferromagnetism
, antiferromagnetism
or ferrimagnetism
. These are properties of the crystal as a whole, of little bearing on chemical properties.
, can be put together. With paramagnetic compounds the observed susceptibility can be adjusted by adding to it the so-called diamagnetic correction, which is the diamagnetic susceptibility calculated with the values from the table.
gives the ratio of the two populations as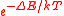 , where k is the Boltzmann constant and T is the temperature in kelvin
, where k is the Boltzmann constant and T is the temperature in kelvin
s. In most cases ΔE is much smaller than kT and the exponential can be expanded as 1 – ΔE/kT. It follows from the presence of 1/T in this expression that the susceptibility is inversely proportional to temperature.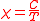
This is known as the Curie law and the proportionality constant, C, is known as the Curie constant, whose value, for molar susceptibility, is calculated as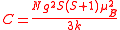
where N is the Avagadro constant, g is the Landé g-factor
, and μB is the Bohr magneton. In this treatment it has been assumed that the electronic ground state
is not degenerate, that the magnetic susceptibility is due only to electron spin and that only the ground state is thermally populated.
While some substances obey the Curie law, others obey the Curie-Weiss law.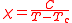
Tc is the Curie temperature. The Curie-Weiss law will apply only when the temperature is well above the Curie temperature. At temperatures below the Curie temperature the substance may become ferromagnetic. More complicated behaviour is observed with the heavier transition elements.

The constant is equal to 2.84 when susceptibility is measured in CGI units, 0,801 when in SI units. The unit for μeff is Bohr magneton (μB). Thus for substances that obey the Curie law, the effective magnetic moment is independent of temperature. For other substances μeff is temperature dependent, but the dependence is small if the Curie-Weiss law holds and the Curie temperature is low.
ion. It is easier to observe in compounds of the heavier elements, such as uranyl
compounds.
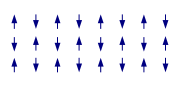 Exchange interactions occur when the substance is not magnetically dilute and there are interactions between individual magnetic centres. One of the simplest systems to exhibit the result of exchange interactions is crystalline copper(II) acetate
Exchange interactions occur when the substance is not magnetically dilute and there are interactions between individual magnetic centres. One of the simplest systems to exhibit the result of exchange interactions is crystalline copper(II) acetate
, Cu2(OAc)4(H2O)2. As the formula indicates, it contains two copper(II) ions. The Cu2+ ions are held together by four acetate ligands, each of which binds to both copper ions. Each Cu2+ ion has a d9 electronic configuration, and so should have one unpaired electron. If there were a covalent bond between the copper ions, the electrons would pair up and the compound would be diamagnetic. Instead, there is an exchange interaction in which the spins of the unpaired electrons become partially aligned to each other. In fact two states are created, one with spins parallel and the other with spins opposed. The energy difference between the two states is so small their populations vary significantly with temperature. In consequence the magnetic moment varies with temperature in a sigmoid
al pattern. The state with spins opposed has lower energy, so the interaction can be classed as antiferromagnetic in this case. It is believed that this is an example of superexchange
, mediated by the oxygen and carbon atoms of the acetate ligands. Other dimers and clusters exhibit exchange behaviour.
Exchange interactions can act over infinite chains in one dimension, planes in two dimensions or over a whole crystal in three dimensions. These are examples of long-range magnetic ordering. They give rise to ferromagnetism
, antiferromagnetism
or ferrimagnetism
, depending on the nature and relative orientations of the individual spins.
Compounds at temperatures below the Curie temperature exhibit long-range magnetic order in the form of ferromagnetism. Another critical temperature is the Néel temperature
, below which ferrimagnetism occurs. The hexahydrate of nickel chloride, NiCl2·6H2O, has a Néel temperature of 8.3 K. The susceptibility is a maximum at this temperature. Below the Néel temperature the susceptibilty decreases and the substance becomes antiferromagnetic.
of the unpaired electrons,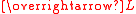 and
and 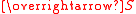 , respectively. "Total" in this context means "vector sum". In the approximation that the electronic states of the metal ions are determined by Russel-Saunders
, respectively. "Total" in this context means "vector sum". In the approximation that the electronic states of the metal ions are determined by Russel-Saunders
coupling and that spin-orbit coupling is negligible, the magnetic moment is given by
certain rotations are not possible. In that case the orbital angular momentum is said to be "quenched" and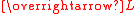 is smaller than might be expected (partial quenching), or zero (complete quenching). There is complete quenching in the following cases. Note that an electron in a degenerate pair of dx2–y2 or dz2 orbitals cannot rotate into the other orbital because of symmetry.
is smaller than might be expected (partial quenching), or zero (complete quenching). There is complete quenching in the following cases. Note that an electron in a degenerate pair of dx2–y2 or dz2 orbitals cannot rotate into the other orbital because of symmetry.
When orbital angular momentum is completely quenched, and the paramagnetism can be attributed to electron spin alone. The total spin angular momentum is simply half the number of unpaired electrons and the spin-only formula results.
and the paramagnetism can be attributed to electron spin alone. The total spin angular momentum is simply half the number of unpaired electrons and the spin-only formula results.
where n is the number of unpaired electrons. The spin-only formula is a good first approximation for high-spin complexes of first-row transition metal
s.
The small deviations from the spin-only formula may result from the neglect of orbital angular momentum or of spin-orbit coupling. For example, tetrahedral d3, d4, d8 and d9 complexes tend to show larger deviations from the spin-only formula than octahedral complexes of the same ion, because "quenching" of the orbital contribution is less effective in the tetrahedral case.
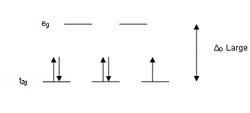
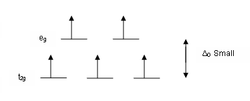 According to crystal field theory, the d orbitals of a transition metal ion in an octahedal complex are split into two groups in a crystal field. If the splitting is large enough to overcome the energy needed to place electrons in the same orbital, with opposite spin, a low-spin complex will result.
According to crystal field theory, the d orbitals of a transition metal ion in an octahedal complex are split into two groups in a crystal field. If the splitting is large enough to overcome the energy needed to place electrons in the same orbital, with opposite spin, a low-spin complex will result.
With one unpaired electron μeff values range from 1.8 to 2.5 μB and with two unpaired electrons the range is 3.18 to 3.3 μB. Note that low-spin complexes of Fe2+ and Co3+ are diamagnetic. Another group of complexes that are diamagnetic are square-planar complexes of d8 ions such as Ni2+ and Rh+ and Au3+.
iron(III), Fe(S2CNR2)3, is a well-documented example. The effective moment varies from a typical d5 low-spin value of 2.25 μB at 80 K to more than 4 μB above 300 K.
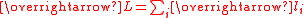
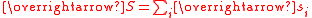

and the calculated magnetic moment is given by
In actinides spin-orbit coupling is strong and the coupling approximates to j j coupling.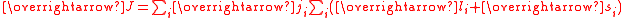
This means that it is difficult to calculate the effective moment. For example, uranium(IV), f2, in the complex [UCl6]2– has a measured effective moment of 2.2 μB, which includes a contribution from temperature-independent paramagnetism.
, O2; nitric oxide
, NO; nitrogen dioxide
, NO2 and chlorine dioxide
, ClO2. In organic chemistry
, compounds with an unpaired electron are said to be free radicals. Free radicals, with some exceptions, are short-lived because one free radical will react rapidly with another, so their magnetic properties are difficult to study. However, if the radicals are well separated from each other in a dilute solution in a solid matrix, at low temperature, they can be studied by electron paramagnetic resonance
(EPR). Such radicals are generated by irradiation. Extensive EPR studies have revealed much about electron delocalization in free radicals. The simulated spectrum of the CH3• radical shows hyperfine splitting due to the interaction of the electron with the 3 equivalent hydrogen nuclei, each of which has a spin of 1/2.
Spin label
s are long-lived free radicals which can be inserted into organic molecules so that they can be studied by EPR.
For example, the nitroxide MTSL
, a functionalized derivative of TEtra Methyl Piperidine Oxide, TEMPO
, is used in site-directed spin labeling
.
ion, Gd3+, has the f7 electronic configuration, with all spins parallel. Compounds of the Gd3+ ion are the most suitable for use as a contrast agent
for MRI scans. The magnetic moments of gadolinium compounds are larger than those of any transition metal ion. Gadolinium is
preferred to other lanthanide ions, some of which have larger effective moments, due to its having a non-degenerate
electronic ground state
.
For many years the nature of oxyhemoglobin, Hb-O2, was highly controversial. It was found experimentally to be diamagnetic. Deoxy-hemoglobin is generally accepted to be a complex of iron in the +2 oxidation state
, that is a d6 system with a high-spin magnetic moment near to the spin-only value of 4.9 μB. It was proposed that the iron is oxidized and the oxygen reduced to superoxide.
Pairing up of electrons from Fe3+ and O2– was then proposed to occur via an exchange mechanism. It has now been shown that in fact the iron(II) changes from high-spin to low-spin when an oxygen molecule donates a pair of electrons to the iron. Whereas in deoxy-hemoglobin the iron atom lies above the plane of the heme, in the low-spin complex the effective ionic radius
is reduced and the iron atom lies in the heme plane.
This information has an important bearing on research to find artificial oxygen carriers.
Compounds of gallium(II) were unknown until quite recently. As the atomic number of gallium is an odd number (31), Ga2+ should have an unpaired electron. It was assumed that it would act as a free radical and have a very short lifetime. The non-existence of Ga(II) compounds was part of the so-called inert pair effect
. When salts of the anion with empirical formula
such as [GaCl3]– were synthesized they were found to be diamagnetic. This implied the formation of a Ga-Ga bond and a dimeric formula, [Ga2Cl6]2–.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons
Electron pair
In chemistry, an electron pair consists of two electrons that occupy the same orbital but have opposite spins.Because electrons are fermions, the Pauli exclusion principle forbids these particles from having exactly the same quantum numbers. Therefore the only way to occupy the same orbital, i.e....
. Molecular compounds that contain one or more unpaired electron
Unpaired electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. As the formation of electron pairs is often energetically favourable, either in the form of a chemical bond or as a lone pair, unpaired electrons are relatively...
s are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s and actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
, antiferromagnetism
Antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usuallyrelated to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism...
or ferrimagnetism
Ferrimagnetism
In physics, a ferrimagnetic material is one in which the magnetic moments of the atoms on different sublattices are opposed, as in antiferromagnetism; however, in ferrimagnetic materials, the opposing moments are unequal and a spontaneous magnetization remains...
depending on the relative orientations of the individual spins.
Magnetic susceptibility
The primary measurement in magnetochemistry is magnetic susceptibility. This measures the strength of interaction on placing the substance in a magnetic field. The volume magnetic susceptibility, represented by the symbol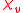 is defined by the relationship
is defined by the relationship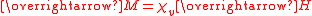
where,
 is the magnetization
is the magnetizationMagnetization
In classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
of the material (the magnetic dipole moment per unit volume), measured in ampere
Ampere
The ampere , often shortened to amp, is the SI unit of electric current and is one of the seven SI base units. It is named after André-Marie Ampère , French mathematician and physicist, considered the father of electrodynamics...
s per meter ( SI
International System of Units
The International System of Units is the modern form of the metric system and is generally a system of units of measurement devised around seven base units and the convenience of the number ten. The older metric system included several groups of units...
units), and
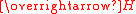 is the magnetic field strength, also measured in amperes per meter. Susceptibility is a dimensionless quantity
is the magnetic field strength, also measured in amperes per meter. Susceptibility is a dimensionless quantityDimensionless quantity
In dimensional analysis, a dimensionless quantity or quantity of dimension one is a quantity without an associated physical dimension. It is thus a "pure" number, and as such always has a dimension of 1. Dimensionless quantities are widely used in mathematics, physics, engineering, economics, and...
. For chemical applications the molar magnetic susceptibility (χmol) is the preferred quantity. It is measured in m3·mol−1 (SI) or cm3·mol−1 (CGS) and is defined as

where ρ is the density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
in kg·m−3 (SI) or g·cm−3 (CGS) and M is molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
in kg·mol−1 (SI) or g·mol−1 (CGS).
A variety of methods are available for the measurement of magnetic susceptibility.
- With the Gouy balance the weight change of the sample is measured with an analytical balance when the sample is placed in a homogeneous magnetic field. The measurements are calibrated against a known standard, such as mercury cobalt thiocyanate, HgCo(NCS)4. Calibration removes the need to know the density of the sample. Variable temperature measurements can be made by placing the sample in a cryostatCryostatA cryostat is a device used to maintain cold cryogenic temperatures. Low temperatures may be maintained within a cryostat by using various refrigeration methods, most commonly using cryogenic fluid bath such as liquid helium. Hence it is usually assembled into a vessel, similar in construction...
between the pole pieces of the magnet.
- The Evans balanceEvans balanceAn Evans balance is a device for measuring magnetic susceptibility. Magnetic susceptibility is related to the force experienced by a substance in a magnetic field...
. is a torsion balance which uses a sample in a fixed position and a variable secondary magnet to bring the magnets back to their initial position. It, too, is calibrated against HgCo(NCS)4.
- With a Faraday balanceFaraday balanceA Faraday balance is a device for measuring magnetic susceptibility. Magnetic susceptibility is related to the force experienced by a substance in a magnetic field...
the sample is placed in a magnetic field of constant gradient, and weighed on a torsion balance. This method can yield information on magnetic anisotropyMagnetic anisotropyMagnetic anisotropy is the direction dependence of a material's magnetic properties. In the absence of an applied magnetic field, a magnetically isotropic material has no preferential direction for its magnetic moment while a magnetically anisotropic material will align its moment with one of the...
.
- SQUIDSQUIDA SQUID is a very sensitive magnetometer used to measure extremely weak magnetic fields, based on superconducting loops containing Josephson junctions....
is a very sensitive magnetometer.
- For substances in solution NMRNuclear magnetic resonanceNuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
may be used to measure susceptibility.
Types of magnetic behaviour
When an isolated atom is placed in a magnetic fieldMagnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
there is an interaction because each electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
in the atom behaves like a magnet, that is, the electron has a magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
. There are two type of interaction.
- Diamagnetism. Every electron is paired with another electron in the same atomic orbitalAtomic orbitalAn atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
. The moments of the two electrons cancel each other out, so the atom has no net magnetic moment. When placed in a magnetic field the atom becomes magnetically polarized, that is, it develops an induced magnetic moment. The force of the interaction tends to push the atom out of the magnetic field. By convention diamagnetic susceptibility is given a negative sign. - Paramagnetism. At least one electron is not paired with another. The atom has a permanent magnetic moment. When placed into a magnetic field, the atom is attracted into the field. By convention paramagnetic susceptibility is given a positive sign.
When the atom is present in a chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
its magnetic behaviour is modified by its chemical environment. Measurement of the magnetic moment can give useful chemical information.
In certain crystalline materials individual magnetic moments may be aligned with each other (magnetic moment has both magnitude and direction). This gives rise to ferromagnetism
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
, antiferromagnetism
Antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usuallyrelated to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism...
or ferrimagnetism
Ferrimagnetism
In physics, a ferrimagnetic material is one in which the magnetic moments of the atoms on different sublattices are opposed, as in antiferromagnetism; however, in ferrimagnetic materials, the opposing moments are unequal and a spontaneous magnetization remains...
. These are properties of the crystal as a whole, of little bearing on chemical properties.
Diamagnetism
Diamagnetism is a universal property of chemical compounds, because all chemical compounds contain electron pairs. A compound in which there are no unpaired electrons is said to be diamagnetic. The effect is weak because it depends on the magnitude of the induced magnetic moment. It depends on the number of electron pairs and the chemical nature of the atoms to which they belong. This means that the effects are additive, and a table of "diamagnetic contributions", or Pascal's constantsPascal's constants
Pascals’ constants are numbers used in the evaluation of the magnetic susceptibilities of coordination compounds. The magnetic susceptibility of a compound is the sum of the paramagnetic susceptibility associated with the unpaired electrons and the opposing diamagnetic susceptibility associated...
, can be put together. With paramagnetic compounds the observed susceptibility can be adjusted by adding to it the so-called diamagnetic correction, which is the diamagnetic susceptibility calculated with the values from the table.
Mechanism and temperature dependence
A metal ion with a single unpaired electron, such as Cu2+, in a coordination complex provides the simplest illustration of the mechanism of paramagnetism. The individual metal ions are kept far apart by the ligands, so that there is no magnetic interaction between them. The system is said to be magnetically dilute. The magnetic dipoles of the atoms point in random directions. When a magnetic field is applied, first-order Zeeman splitting occurs. Atoms with spins aligned to the field slightly outnumber the atoms with non-aligned spins. In the first-order Zeeman effect the energy difference between the two states is proportional to the applied field strength. Denoting the energy difference as ΔE, the Boltzmann distributionBoltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
gives the ratio of the two populations as
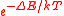 , where k is the Boltzmann constant and T is the temperature in kelvin
, where k is the Boltzmann constant and T is the temperature in kelvinKelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
s. In most cases ΔE is much smaller than kT and the exponential can be expanded as 1 – ΔE/kT. It follows from the presence of 1/T in this expression that the susceptibility is inversely proportional to temperature.
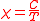
This is known as the Curie law and the proportionality constant, C, is known as the Curie constant, whose value, for molar susceptibility, is calculated as
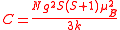
where N is the Avagadro constant, g is the Landé g-factor
Landé g-factor
In physics, the Landé g-factor is a particular example of a g-factor, namely for an electron with both spin and orbital angular momenta. It is named after Alfred Landé, who first described it in 1921....
, and μB is the Bohr magneton. In this treatment it has been assumed that the electronic ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
is not degenerate, that the magnetic susceptibility is due only to electron spin and that only the ground state is thermally populated.
While some substances obey the Curie law, others obey the Curie-Weiss law.
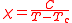
Tc is the Curie temperature. The Curie-Weiss law will apply only when the temperature is well above the Curie temperature. At temperatures below the Curie temperature the substance may become ferromagnetic. More complicated behaviour is observed with the heavier transition elements.
Effective magnetic moment
When the Curie law is obeyed, the product of susceptibility and temperature is a constant. The effective magnetic moment, μeff is then calculated as
The constant is equal to 2.84 when susceptibility is measured in CGI units, 0,801 when in SI units. The unit for μeff is Bohr magneton (μB). Thus for substances that obey the Curie law, the effective magnetic moment is independent of temperature. For other substances μeff is temperature dependent, but the dependence is small if the Curie-Weiss law holds and the Curie temperature is low.
Temperature independent paramagnetism
Compounds which are expected to be diamagnetic may exhibit this kind of weak paramagnetism. It arises from a second-order Zeeman effect in which additional splitting, proportional to the square of the field strength, occurs. It is difficult to observe as the compound inevitably also interacts with the magnetic field in the diamagnetic sense. Nevertheless, data are available for the permanganatePermanganate
A permanganate is the general name for a chemical compound containing the manganate ion, . Because manganese is in the +7 oxidation state, the permanganate ion is a strong oxidizing agent. The ion has tetrahedral geometry...
ion. It is easier to observe in compounds of the heavier elements, such as uranyl
Uranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula [UO2]2+. It has a linear structure with short U-O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands are bound to the uranyl ion in an equatorial plane...
compounds.
Exchange interactions


Copper(II) acetate
Copper acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu2 where OAc- is acetate . The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu2 is a dark green crystalline solid, whereas Cu22 is...
, Cu2(OAc)4(H2O)2. As the formula indicates, it contains two copper(II) ions. The Cu2+ ions are held together by four acetate ligands, each of which binds to both copper ions. Each Cu2+ ion has a d9 electronic configuration, and so should have one unpaired electron. If there were a covalent bond between the copper ions, the electrons would pair up and the compound would be diamagnetic. Instead, there is an exchange interaction in which the spins of the unpaired electrons become partially aligned to each other. In fact two states are created, one with spins parallel and the other with spins opposed. The energy difference between the two states is so small their populations vary significantly with temperature. In consequence the magnetic moment varies with temperature in a sigmoid
Sigmoid
Sigmoid means resembling the lower-case Greek letter sigma or the Latin letter S. Specific uses include:* Sigmoid function, a mathematical function* Sigmoid colon, part of the large intestine or colon...
al pattern. The state with spins opposed has lower energy, so the interaction can be classed as antiferromagnetic in this case. It is believed that this is an example of superexchange
Superexchange
Superexchange is the strong antiferromagnetic coupling between two next-to-nearest neighbor cations through a non-magnetic anion. In this way, it differs from direct exchange in which there is coupling between nearest neighbor cations not involving an intermediary anion...
, mediated by the oxygen and carbon atoms of the acetate ligands. Other dimers and clusters exhibit exchange behaviour.
Exchange interactions can act over infinite chains in one dimension, planes in two dimensions or over a whole crystal in three dimensions. These are examples of long-range magnetic ordering. They give rise to ferromagnetism
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
, antiferromagnetism
Antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usuallyrelated to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism...
or ferrimagnetism
Ferrimagnetism
In physics, a ferrimagnetic material is one in which the magnetic moments of the atoms on different sublattices are opposed, as in antiferromagnetism; however, in ferrimagnetic materials, the opposing moments are unequal and a spontaneous magnetization remains...
, depending on the nature and relative orientations of the individual spins.
Compounds at temperatures below the Curie temperature exhibit long-range magnetic order in the form of ferromagnetism. Another critical temperature is the Néel temperature
Néel temperature
The Néel temperature or magnetic ordering temperature , TN, is the temperature above which an antiferromagnetic material becomes paramagnetic—that is, the thermal energy becomes large enough to destroy the macroscopic magnetic ordering within the material....
, below which ferrimagnetism occurs. The hexahydrate of nickel chloride, NiCl2·6H2O, has a Néel temperature of 8.3 K. The susceptibility is a maximum at this temperature. Below the Néel temperature the susceptibilty decreases and the substance becomes antiferromagnetic.
Theoretical calculation for complexes of metal ions
The effective magnetic moment for a compound containing a metal ion with one or more unpaired electrons depends on the total orbital and spin angular momentumAngular momentum
In physics, angular momentum, moment of momentum, or rotational momentum is a conserved vector quantity that can be used to describe the overall state of a physical system...
of the unpaired electrons,
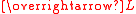 and
and 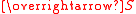 , respectively. "Total" in this context means "vector sum". In the approximation that the electronic states of the metal ions are determined by Russel-Saunders
, respectively. "Total" in this context means "vector sum". In the approximation that the electronic states of the metal ions are determined by Russel-SaundersAngular momentum coupling
In quantum mechanics, the procedure of constructing eigenstates of total angular momentum out of eigenstates of separate angular momenta is called angular momentum coupling. For instance, the orbit and spin of a single particle can interact through spin-orbit interaction, in which case the...
coupling and that spin-orbit coupling is negligible, the magnetic moment is given by

Spin-only formula
Orbital angular momentum is generated when an electron in an orbital of a degenerate set of orbitals is moved to another orbital in the set by rotation. In complexes of high symmetryMolecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
certain rotations are not possible. In that case the orbital angular momentum is said to be "quenched" and
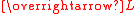 is smaller than might be expected (partial quenching), or zero (complete quenching). There is complete quenching in the following cases. Note that an electron in a degenerate pair of dx2–y2 or dz2 orbitals cannot rotate into the other orbital because of symmetry.
is smaller than might be expected (partial quenching), or zero (complete quenching). There is complete quenching in the following cases. Note that an electron in a degenerate pair of dx2–y2 or dz2 orbitals cannot rotate into the other orbital because of symmetry.
| dn | Octahedral | Tetrahedral | ||
| high-spin | low-spin | |||
| d1 | e1 | |||
| d2 | e2 | |||
| d3 | t2g3 | |||
| d4 | t2g3eg1 | |||
| d5 | t2g3eg2 | |||
| d6 | t2g6 | e3t23 | ||
| d7 | t2g6eg1 | e4t23 | ||
| d8 | t2g6eg2 | |||
| d9 | t2g6eg3 |
- legend: t2g, t2 = (dxy, dxz, dyz). eg, e = (dx2–y2, dz2).
When orbital angular momentum is completely quenched,
 and the paramagnetism can be attributed to electron spin alone. The total spin angular momentum is simply half the number of unpaired electrons and the spin-only formula results.
and the paramagnetism can be attributed to electron spin alone. The total spin angular momentum is simply half the number of unpaired electrons and the spin-only formula results.
where n is the number of unpaired electrons. The spin-only formula is a good first approximation for high-spin complexes of first-row transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s.
| Ion | Number of unpaired electrons | Spin-only moment /μB | observed moment /μB |
|---|---|---|---|
| Ti3+ | 1 | 1.73 | 1.73 |
| V4+ | 1 | 1.68–1.78 | |
| Cu2+ | 1 | 1.70–2.20 | |
| V3+ | 2 | 2.83 | 2.75–2.85 |
| Ni2+ | 2 | 2.8–3.5 | |
| V2+ | 3 | 3.88 | 3.80–3.90 |
| Cr3+ | 3 | 3.70–3.90 | |
| Co2+ | 3 | 4.3–5.0 | |
| Mn4+ | 3 | 3.80–4.0 | |
| Cr2+ | 4 | 4.90 | 4.75–4.90 |
| Fe2+ | 4 | 5.1–5.7 | |
| Mn2+ | 5 | 5.92 | 5.65–6.10 |
| Fe3+ | 5 | 5.7–6.0 |
The small deviations from the spin-only formula may result from the neglect of orbital angular momentum or of spin-orbit coupling. For example, tetrahedral d3, d4, d8 and d9 complexes tend to show larger deviations from the spin-only formula than octahedral complexes of the same ion, because "quenching" of the orbital contribution is less effective in the tetrahedral case.
Low-spin complexes


| d-count | Number of unpaired electrons | examples | |
|---|---|---|---|
| high-spin | low-spin | ||
| d4 | 4 | 2 | Cr2+ , Mn3+ |
| d5 | 5 | 1 | Mn2+, Fe3+ |
| d6 | 4 | 0 | Fe2+, Co3+ |
| d7 | 3 | 1 | Co2+ |
With one unpaired electron μeff values range from 1.8 to 2.5 μB and with two unpaired electrons the range is 3.18 to 3.3 μB. Note that low-spin complexes of Fe2+ and Co3+ are diamagnetic. Another group of complexes that are diamagnetic are square-planar complexes of d8 ions such as Ni2+ and Rh+ and Au3+.
Spin cross-over
When the energy difference between the high-spin and low-spin states is comparable to kT (k is the Boltzmann constant and T the temperature) an equilibrium is established between the spin states, involving what have been called "electronic isomers". Tris-dithiocarbamatoDithiocarbamate
A dithiocarbamate is a functional group in organic chemistry. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms. Sodium diethyldithiocarbamate is a common ligand in inorganic chemistry....
iron(III), Fe(S2CNR2)3, is a well-documented example. The effective moment varies from a typical d5 low-spin value of 2.25 μB at 80 K to more than 4 μB above 300 K.
2nd and 3rd row transition metals
Crystal field splitting is larger for complexes of the heavier transition metals than for the transition metals discussed above. A consequence of this is that low-spin complexes are much more common. Spin-orbit coupling constants, ζ, are also larger and cannot be ignored, even in elementary treatments. The magnetic behaviour has been summarized, as below, together with an extensive table of data.| d-count | kT/ζ=0.1 μeff | kT/ζ=0 μeff | Behaviour with large spin-orbit coupling constant, ζnd |
|---|---|---|---|
| d1 | 0.63 | 0 | μeff varies with T1/2 |
| d2 | 1.55 | 1.22 | μeff varies with T, approximately |
| d3 | 3.88 | 3.88 | Independent of temperature |
| d4 | 2.64 | 0 | μeff varies with T1/2 |
| d5 | 1.95 | 1.73 | μeff varies with T, approximately |
Lanthanides and actinides
Russel-Saunders coupling, LS coupling, applies to the lanthanide ions, crystal field effects can be ignored, but spin-orbit coupling is not negligible. Consequently spin and orbital angular momenta have to be combined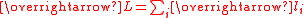
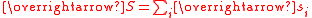

and the calculated magnetic moment is given by

| lanthanide | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of unpaired électrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| calculated moment /μB | 2.54 | 3.58 | 3.62 | 2.68 | 0.85 | 0 | 7.94 | 9.72 | 10.65 | 10.6 | 9.58 | 7.56 | 4.54 | 0 |
| observed moment /μB | 2.3–2.5 | 3.4–3.6 | 3.5–3.6 | 1.4–1.7 | 3.3–3.5 | 7.9–8.0 | 9.5–9.8 | 10.4–10.6 | 10.4–10.7 | 9.4–9.6 | 7.1–7.5 | 4.3–4.9 | 0 |
In actinides spin-orbit coupling is strong and the coupling approximates to j j coupling.
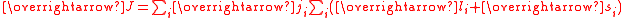
This means that it is difficult to calculate the effective moment. For example, uranium(IV), f2, in the complex [UCl6]2– has a measured effective moment of 2.2 μB, which includes a contribution from temperature-independent paramagnetism.
Main group elements and organic compounds
Very few compounds of main group elements are paramagnetic. Notable examples include: oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, O2; nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
, NO; nitrogen dioxide
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
, NO2 and chlorine dioxide
Chlorine dioxide
Chlorine dioxide is a chemical compound with the formula ClO2. This yellowish-green gas crystallizes as bright orange crystals at −59 °C. As one of several oxides of chlorine, it is a potent and useful oxidizing agent used in water treatment and in bleaching....
, ClO2. In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, compounds with an unpaired electron are said to be free radicals. Free radicals, with some exceptions, are short-lived because one free radical will react rapidly with another, so their magnetic properties are difficult to study. However, if the radicals are well separated from each other in a dilute solution in a solid matrix, at low temperature, they can be studied by electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
(EPR). Such radicals are generated by irradiation. Extensive EPR studies have revealed much about electron delocalization in free radicals. The simulated spectrum of the CH3• radical shows hyperfine splitting due to the interaction of the electron with the 3 equivalent hydrogen nuclei, each of which has a spin of 1/2.
Spin label
Spin label
A spin label is an organic molecule which possesses an unpaired electron, usually on a nitrogen atom, and the ability to bind to another molecule. Spin labels are normally used as tools for probing proteins or biological membrane-local dynamics using EPR spectroscopy. The site-directed spin...
s are long-lived free radicals which can be inserted into organic molecules so that they can be studied by EPR.
For example, the nitroxide MTSL
MTSL
MTSL is a chemical compound which can be used as a nitroxide paramagnetic spin label in protein Electron paramagnetic resonance spectroscopy experiments. MTSL is attached via a disulfide bond to a cysteine residue, enabling site-directed spin labelling...
, a functionalized derivative of TEtra Methyl Piperidine Oxide, TEMPO
TEMPO
oxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...
, is used in site-directed spin labeling
Site-directed spin labeling
Site-directed spin labeling is a technique for investigating protein local dynamics using electron spin resonance. The theory of SDSL is based on the specific reaction of spin labels with amino acids. A spin label's built-in protein structure can be detected by EPR spectroscopy...
.
Applications
The gadoliniumGadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. It is a silvery-white, malleable and ductile rare-earth metal. It is found in nature only in combined form. Gadolinium was first detected spectroscopically in 1880 by de Marignac who separated its oxide and is credited with...
ion, Gd3+, has the f7 electronic configuration, with all spins parallel. Compounds of the Gd3+ ion are the most suitable for use as a contrast agent
MRI contrast agent
MRI contrast agents are a group of contrast media used to improve the visibility of internal body structures in magnetic resonance imaging . The most commonly used compounds for contrast enhancement are gadolinium-based. MRI contrast agents alter the relaxation times of tissues and body cavities...
for MRI scans. The magnetic moments of gadolinium compounds are larger than those of any transition metal ion. Gadolinium is
preferred to other lanthanide ions, some of which have larger effective moments, due to its having a non-degenerate
Degenerate energy level
In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
electronic ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
.
For many years the nature of oxyhemoglobin, Hb-O2, was highly controversial. It was found experimentally to be diamagnetic. Deoxy-hemoglobin is generally accepted to be a complex of iron in the +2 oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
, that is a d6 system with a high-spin magnetic moment near to the spin-only value of 4.9 μB. It was proposed that the iron is oxidized and the oxygen reduced to superoxide.
- Fe(II)Hb (high-spin) + O2 [Fe(III)Hb]O2–
Pairing up of electrons from Fe3+ and O2– was then proposed to occur via an exchange mechanism. It has now been shown that in fact the iron(II) changes from high-spin to low-spin when an oxygen molecule donates a pair of electrons to the iron. Whereas in deoxy-hemoglobin the iron atom lies above the plane of the heme, in the low-spin complex the effective ionic radius
Ionic radius
Ionic radius, rion, is the radius of an atom's ion. Although neither atoms nor ions have sharp boundaries, it is important to treat them as if they are hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice...
is reduced and the iron atom lies in the heme plane.
- Fe(II)Hb + O2 [Fe(II)Hb]O2 (low-spin)
This information has an important bearing on research to find artificial oxygen carriers.
Compounds of gallium(II) were unknown until quite recently. As the atomic number of gallium is an odd number (31), Ga2+ should have an unpaired electron. It was assumed that it would act as a free radical and have a very short lifetime. The non-existence of Ga(II) compounds was part of the so-called inert pair effect
Inert pair effect
The inert pair effect is the tendency of the outermost s electrons to remain nonionized or unshared in compounds of post-transition metals. The term inert pair effect is often used in relation to the increasing stability of oxidation states that are 2 less than the group valency for the heavier...
. When salts of the anion with empirical formula
Empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms of each element present in a compound. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The empirical formula is used as standard for most ionic...
such as [GaCl3]– were synthesized they were found to be diamagnetic. This implied the formation of a Ga-Ga bond and a dimeric formula, [Ga2Cl6]2–.
See also
- Magnetic mineralogyMagnetic mineralogyMagnetic mineralogy is the study of the magnetic properties of minerals. The contribution of a mineral to the total magnetism of a rock depends strongly on the type of magnetic order or disorder. Magnetically disordered minerals contribute a weak magnetism and have no remanence...
- MagnetoelectrochemistryMagnetoelectrochemistryMagnetoelectrochemistry is a branch of electrochemistry dealing with magnetic effects in electrochemistry.-History:These effects have been supposed to exist since the time of Michael Faraday....
- Magnetic ionic liquidMagnetic ionic liquidA magnetic ionic liquid was identified by Satoshi Hayashi and Hiro-o Hamaguchi of the University of Tokyo in 2004 as an ionic liquid based on the imidazole 1-butyl-3-methylimidazolium chloride and ferric chloride....
- Spin iceSpin iceA spin ice is a substance that is similar to water ice in that it can never be completely frozen. This is because it does not have a single minimal-energy state. A spin ice has "spin" degrees of freedom , with frustrated interactions which prevent it freezing...
- Spin glassSpin glassA spin glass is a magnet with frustrated interactions, augmented by stochastic disorder, where usually ferromagnetic and antiferromagnetic bonds are randomly distributed...
- SuperdiamagnetismSuperdiamagnetismSuperdiamagnetism is a phenomenon occurring in certain materials at low temperatures, characterised by the complete absence of magnetic permeability and the exclusion of the interior magnetic field. Superdiamagnetism is a feature of superconductivity...
, SuperparamagnetismSuperparamagnetismSuperparamagnetism is a form of magnetism, which appears in small ferromagnetic or ferrimagnetic nanoparticles. In sufficiently small nanoparticles, magnetization can randomly flip direction under the influence of temperature. The typical time between two flips is called the Néel relaxation time...
, SuperferromagnetismSuperferromagnetismSuperferromagnetism is the magnetism of an ensemble of magnetically interacting super-moment-bearing material particles that would be superparamagnetic if they were not interacting. Nanoparticles of iron oxides, such as ferrihydrite , often cluster and interact magnetically...

