
Electron paramagnetic resonance
Encyclopedia
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy
is a technique for studying chemical species
that have one or more unpaired electron
s, such as organic and inorganic free radicals or inorganic
complexes
possessing a transition metal
ion
. The basic physical concepts of EPR are analogous to those of nuclear magnetic resonance
(NMR), but it is electron spins that are excited instead of spin
s of atomic nuclei
. Because most stable molecules have all their electrons paired, the EPR technique is less widely used than NMR. However, this limitation to paramagnetic species also means that the EPR technique is one of great specificity, since ordinary chemical solvents and matrices do not give rise to EPR spectra.
EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky
in 1944, and was developed independently at the same time by Brebis Bleaney
at the University of Oxford.

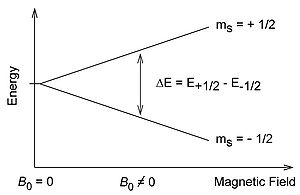
and spin
quantum number
s = 1/2, with magnetic components ms = +1/2 and ms = −1/2. In the presence of an external magnetic field with strength B0, the electron's magnetic moment aligns itself either parallel (ms = −1/2) or antiparallel (ms = +1/2) to the field, each alignment having a specific energy (see the Zeeman effect
). The parallel alignment corresponds to the lower energy state, and the separation between it and the upper state is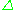 E = geμBB0, where ge is the electron's so-called g-factor (see also the Landé g-factor
E = geμBB0, where ge is the electron's so-called g-factor (see also the Landé g-factor
) and μB is the Bohr magneton. This equation implies that the splitting of the energy levels is directly proportional to the magnetic field
's strength, as shown in the diagram below.
 An unpaired electron can move between the two energy levels by either absorbing or emitting electromagnetic radiation of energy
An unpaired electron can move between the two energy levels by either absorbing or emitting electromagnetic radiation of energy  = h
= h such that the resonance condition,
such that the resonance condition,  =
=  E, is obeyed. Substituting in
E, is obeyed. Substituting in  = h
= h and
and  E = geμBB0 leads to the fundamental equation of EPR spectroscopy: h
E = geμBB0 leads to the fundamental equation of EPR spectroscopy: h = geμBB0. Experimentally, this equation permits a large combination of frequency and magnetic field values, but the great majority of EPR measurements are made with microwaves in the 9000–10000 MHz (9–10 GHz) region, with fields corresponding to about 3500 G (0.35 T). See below for other field-frequency combinations.
= geμBB0. Experimentally, this equation permits a large combination of frequency and magnetic field values, but the great majority of EPR measurements are made with microwaves in the 9000–10000 MHz (9–10 GHz) region, with fields corresponding to about 3500 G (0.35 T). See below for other field-frequency combinations.
In principle, EPR spectra can be generated by either varying the photon frequency incident on a sample while holding the magnetic field constant or doing the reverse. In practice, it is usually the frequency that is kept fixed. A collection of paramagnetic centers, such as free radicals, is exposed to microwaves at a fixed frequency. By increasing an external magnetic field, the gap between the ms = +1/2 and ms = −1/2 energy states is widened until it matches the energy of the microwaves, as represented by the double-arrow in the diagram above. At this point the unpaired electrons can move between their two spin states. Since there typically are more electrons in the lower state, due to the Maxwell-Boltzmann distribution (see below), there is a net absorption of energy, and it is this absorption that is monitored and converted into a spectrum.
As an example of how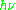 = geμBB0 can be used, consider the case of a free electron, which has ge = 2.0023, and the simulated spectrum shown at the right in two different forms. For the microwave frequency of 9388.2 MHz, the predicted resonance position is a magnetic field of about B0 = h
= geμBB0 can be used, consider the case of a free electron, which has ge = 2.0023, and the simulated spectrum shown at the right in two different forms. For the microwave frequency of 9388.2 MHz, the predicted resonance position is a magnetic field of about B0 = h / geμB = 0.3350 tesla = 3350 gauss, as shown. Note that while two forms of the same spectrum are presented in the figure, most EPR spectra are recorded and published only as first derivatives.
/ geμB = 0.3350 tesla = 3350 gauss, as shown. Note that while two forms of the same spectrum are presented in the figure, most EPR spectra are recorded and published only as first derivatives.
Because of electron-nuclear mass differences, the magnetic moment
of an electron is substantially larger than the corresponding quantity for any nucleus, so that a much higher electromagnetic frequency is needed to bring about a spin resonance with an electron than with a nucleus, at identical magnetic field strengths. For example, for the field of 3350 G shown at the right, spin resonance occurs near 9388.2 MHz for an electron compared to only about 14.3 MHz for 1H nuclei. (For NMR spectroscopy, the corresponding resonance equation is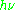 = gNμNB0 where gN and μN depend on the nucleus under study.)
= gNμNB0 where gN and μN depend on the nucleus under study.)
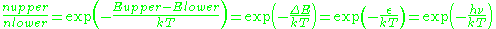
where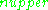 is the number of paramagnetic centers occupying the upper energy state,
is the number of paramagnetic centers occupying the upper energy state, 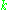 is the Boltzmann constant, and
is the Boltzmann constant, and 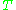 is the temperature in kelvin
is the temperature in kelvin
s. At 298 K, X-band microwave frequencies ( ≈ 9.75 GHz) give
≈ 9.75 GHz) give 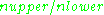 ≈ 0.998, meaning that the upper energy level has a smaller population than the lower one. Therefore, transitions from the lower to the higher level are more probable than the reverse, which is why there is a net absorption of energy.
≈ 0.998, meaning that the upper energy level has a smaller population than the lower one. Therefore, transitions from the lower to the higher level are more probable than the reverse, which is why there is a net absorption of energy.
The sensitivity of the EPR method (i.e., the minimum number of detectable spins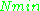 ) depends on the photon frequency
) depends on the photon frequency  according to
according to
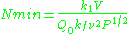
where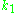 is a constant,
is a constant, 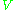 is the sample's volume,
is the sample's volume, 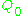 is the unloaded quality factor
is the unloaded quality factor
of the microwave cavity (sample chamber),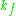 is the cavity filling coefficient, and
is the cavity filling coefficient, and 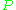 is the microwave power in the spectrometer cavity. With
is the microwave power in the spectrometer cavity. With 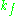 and
and 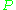 being constants,
being constants, 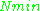 ~
~ 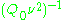 , i.e.,
, i.e., 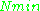 ~
~ 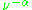 , where
, where  ≈ 1.5. In practice,
≈ 1.5. In practice,  can change varying from 0.5 to 4.5 depending on spectrometer characteristics, resonance conditions, and sample size. In other words, the higher the spectrometer frequency the lower the detection limit (
can change varying from 0.5 to 4.5 depending on spectrometer characteristics, resonance conditions, and sample size. In other words, the higher the spectrometer frequency the lower the detection limit (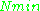 ), meaning greater sensitivity.
), meaning greater sensitivity.
can give information about a paramagnetic center's electronic structure. An unpaired electron responds not only to a spectrometer's applied magnetic field B0 but also to any local magnetic fields of atoms or molecules. The effective field Beff experienced by an electron is thus written
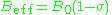
where includes the effects of local fields (
includes the effects of local fields ( can be positive or negative). Therefore, the h
can be positive or negative). Therefore, the h = geμBBeff resonance condition (above) is rewritten as follows:
= geμBBeff resonance condition (above) is rewritten as follows:
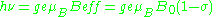
The quantity ge(1 – σ) is denoted g and called simply the g-factor, so that the final resonance equation becomes
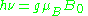
This last equation is used to determine g in an EPR experiment by measuring the field and the frequency at which resonance occurs. If g does not equal ge the implication is that the ratio of the unpaired electron's spin magnetic moment to its angular momentum differs from the free electron value. Since an electron's spin magnetic moment is constant (approximately the Bohr magneton), then the electron must have gained or lost angular momentum through spin-orbit coupling. Because the mechanisms of spin-orbit coupling are well understood, the magnitude of the change gives information about the nature of the atomic or molecular orbital containing the unpaired electron.
In general, the g factor is not a number
but a second-rank tensor
represented by nine numbers arranged in a 3×3 matrix
. The principal axes
of this tensor are determined by the local fields, for example, by the local atomic arrangement around the unpaired spin in a solid or in a molecule. Choosing an appropriate coordinate system (say, x,y,z) allows to "diagonalize" this tensor thereby reducing the maximum number of its components from nine to three, gxx, gyy and gzz. For a single spin experiencing only Zeeman interaction with an external magnetic field, the position of the EPR resonance is given by the expression gxxBx + gyyBy + gzzBz. Here Bx, By and Bz are the components of the magnetic field vector in the coordinate system (x,y,z); their magnitudes change as the field is rotated, so as the frequency of the resonance. For a large ensemble of randomly oriented spins, the EPR spectrum consists three peaks of characteristic shape at frequencies gxxB0, gyyB0 and gzzB0: the low-frequency peak is positive in first-derivative spectra, the high-frequency peak is negative, and the central peak is bipolar. Such situation is commonly observed in powders and the spectra are therefore called "powder-pattern spectra". In crystals, the number of EPR lines is determined by the number of crystallographically equivalent orientations of the EPR spin (called "EPR center").
Two common mechanisms by which electrons and nuclei interact are the Fermi contact interaction
and by dipolar interaction. The former applies largely to the case of isotropic interactions (independent of sample orientation in a magnetic field) and the latter to the case of anisotropic interactions (spectra dependent on sample orientation in a magnetic field). Spin polarization is a third mechanism for interactions between an unpaired electron and a nuclear spin, being especially important for -electron organic radicals, such as the benzene radical anion. The symbols "a" or "A" are used for isotropic hyperfine coupling constants while "B" is usually employed for anisotropic hyperfine coupling constants.
-electron organic radicals, such as the benzene radical anion. The symbols "a" or "A" are used for isotropic hyperfine coupling constants while "B" is usually employed for anisotropic hyperfine coupling constants.
In many cases, the isotropic hyperfine splitting pattern for a radical freely tumbling in a solution (isotropic system) can be predicted.
While it is easy to predict the number of lines a radical's EPR spectrum should show, the reverse problem, unraveling a complex multi-line EPR spectrum and assigning the various spacings to specific nuclei, is more difficult.
In the oft-encountered case of I = 1/2 nuclei (e.g., 1H, 19F, 31P), the line intensities produced by a population of radicals, each possessing M equivalent nuclei, will follow Pascal's triangle
. For example, the spectrum at the right shows that the three 1H nuclei of the CH3 radical give rise to 2MI + 1 = 2(3)(1/2) + 1 = 4 lines with a 1:3:3:1 ratio. The line spacing gives a hyperfine coupling constant of aH = 23 G for each of the three 1H nuclei. Note again that the lines in this spectrum are first derivatives of absorptions.
As a second example, consider the methoxymethyl radical, H2C(OCH3). The two equivalent methyl hydrogens will give an overall 1:2:1 EPR pattern, each component of which is further split by the three methoxy hydrogens into a 1:3:3:1 pattern to give a total of 3×4 = 12 lines, a triplet of quartets. A simulation of the observed EPR spectrum is shown at the right, and agrees with the 12-line prediction and the expected line intensities. Note that the smaller coupling constant (smaller line spacing) is due to the three methoxy hydrogens, while the larger coupling constant (line spacing) is from the two hydrogens bonded directly to the carbon atom bearing the unpaired electron. It is often the case that coupling constants decrease in size with distance from a radical's unpaired electron, but there are some notable exceptions, such as the ethyl radical (CH2CH3).
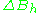 is the distance measured from the line's center to the point in which absorption
is the distance measured from the line's center to the point in which absorption
value has half of maximal absorption value in the center of resonance
line. First inclination width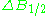 is a distance from center of the line to the point of maximal absorption curve inclination. In practice, a full definition of linewidth is used. For symmetric lines, halfwidth
is a distance from center of the line to the point of maximal absorption curve inclination. In practice, a full definition of linewidth is used. For symmetric lines, halfwidth 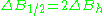 , and full inclination width
, and full inclination width 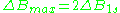
. The spin-lattice
relaxation time can be measured with an inversion recovery experiment.
As with pulsed NMR
, the Hahn echo is central to many pulsed EPR experiments. A Hahn echo
decay experiment can be used to measure the dephasing time, as shown in the animation below. The size of the echo is recorded for different spacings of the two pulses. This reveals the decoherence, which is not refocused by the pulse. In simple cases, an exponential decay is measured, which is described by the
pulse. In simple cases, an exponential decay is measured, which is described by the 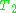 time.
time.
and physics
, for the detection and identification of free radical
s and paramagnetic centers such as F centers
. EPR is a sensitive, specific method for studying both radicals formed in chemical reactions and the reactions themselves. For example, when frozen water (solid H2O) is decomposed by exposure to high-energy radiation, radicals such as H, OH, and HO2 are produced. Such radicals can be identified and studied by EPR. Organic and inorganic radicals can be detected in electrochemical systems and in materials exposed to UV light. In many cases, the reactions to make the radicals and the subsequent reactions of the radicals are of interest, while in other cases EPR is used to provide information on a radical's geometry and the orbital of the unpaired electron.
Medical
and biological
applications of EPR also exist. Although radicals are very reactive, and so do not normally occur in high concentrations in biology, special reagents have been developed to spin-label molecules of interest. These reagents are particularly useful in biological systems. Specially-designed nonreactive radical molecules can attach to specific sites in a biological cell
, and EPR spectra can then give information on the environment of these so-called spin-label
or spin-probes
.
A type of dosimetry system
has been designed for reference standards and routine use in medicine, based on EPR signals of radicals from irradiated polycrystalline α-alanine
(the alanine deamination radical, the hydrogen abstraction radical, and the (CO-(OH))=C(CH3)NH2+ radical) . This method is suitable for measuring gamma
and x-ray
s, electrons, protons, and high-linear energy transfer
(LET) radiation of doses
in the 1 Gy
to 100 kGy range.
EPR spectroscopy can be applied only to systems in which the balance between radical decay and radical formation keeps the free-radicals concentration above the detection limit of the spectrometer used. This can be a particularly severe problem in studying reactions in liquids. An alternative approach is to slow down reactions by studying samples held at cryogenic temperatures, such as 77 K (liquid nitrogen
) or 4.2 K (liquid helium). An example of this work is the study of radical reactions in single crystals of amino acids exposed to x-rays, work that sometimes leads to activation energies
and rate constants for radical reactions.
The study of radiation-induced free radicals in biological substances (for cancer research) poses the additional problem that tissue contains water, and water (due to its electric dipole moment
) has a strong absorption band in the microwave
region used in EPR spectrometers.
EPR also has been used by archaeologists for the dating of teeth. Radiation damage over long periods of time creates free radicals in tooth enamel, which can then be examined by EPR and, after proper calibration, dated. Alternatively, material extracted from the teeth of people during dental procedures can be used to quantify their cumulative exposure to ionizing radiation. People exposed to radiation from the Chernobyl disaster
have been examined by this method.
Radiation-sterilized foods have been examined with EPR spectroscopy, the aim being to develop methods to determine if a particular food sample has been irradiated and to what dose.
Because of its high sensitivity, EPR was used recently to measure the quantity of energy used locally during a mechanochemical milling process.
EPR spectroscopy has been used to measure properties of crude oil, in particular asphaltene and vanadium
content. EPR measurement of asphaltene content is a function of spin density and solvent polarity. Prior work dating to the 1960s has demonstrated the ability to measure vanadium
content to sub-ppm levels.
) in collaboration with L. G. Oranski's group (Ukrainian Physics and Technics Institute, Donetsk), which began working in the Institute of Problems of Chemical Physics
, Chernogolovka
around 1975. Two decades later, a W-band EPR spectrometer was produced as a small commercial line by the German Bruker Company
, initiating the expansion of W-band EPR techniques into medium-sized academic laboratories. Today there still are only a few scientific centers in the world capable of high-field high-frequency EPR; among them are the Grenoble High Magnetic Field Laboratory in Grenoble
, France
, the Physics Department in Freie Universität Berlin, the National High Magnetic Field Laboratory in Tallahassee, US, the National Center for Advanced ESR Technology (ACERT) at Cornell University
in Ithaca
, US, the Department of Physiology, and Biophysics at Albert Einstein College of Medicine
, Bronx, NY, the IFW in Dresden
, Germany
, the Institute of Physics of Complex Matter in Lausanne
in Switzerland
, and the Institute of Physics of the Leiden University
, Netherlands
.
The EPR waveband is stipulated by the frequency or wavelength of a spectrometer's microwave source (see Table).
EPR experiments often are conducted at X
and, less commonly, Q bands, mainly due to the ready availability of the necessary microwave components (which originally were developed for radar applications). A second reason for widespread X and Q band measurements is that electromagnets can reliably generate fields up to about 1 tesla. However, the low spectral resolution over g-factor at these wavebands limits the study of paramagnetic centers with comparatively low anisotropic magnetic parameters. Measurements at > 40 GHz, in the millimeter wavelength region, offer the following advantages:
> 40 GHz, in the millimeter wavelength region, offer the following advantages:
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
is a technique for studying chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
that have one or more unpaired electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s, such as organic and inorganic free radicals or inorganic
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
possessing a transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
. The basic physical concepts of EPR are analogous to those of nuclear magnetic resonance
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
(NMR), but it is electron spins that are excited instead of spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
s of atomic nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. Because most stable molecules have all their electrons paired, the EPR technique is less widely used than NMR. However, this limitation to paramagnetic species also means that the EPR technique is one of great specificity, since ordinary chemical solvents and matrices do not give rise to EPR spectra.
EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky
Yevgeny Zavoisky
Yevgeny Konstantinovich Zavoisky was a Soviet physicist known for discovery of electron paramagnetic resonance in 1944. He likely observed nuclear magnetic resonance in 1941, well before Felix Bloch and Edward Mills Purcell, but dismissed the results as not reproducible...
in 1944, and was developed independently at the same time by Brebis Bleaney
Brebis Bleaney
Brebis Bleaney CBE FRS was a British physicist. His main area of research was the use of microwave techniques to study the magnetic properties of solids. He was head of the Clarendon Laboratory at the University of Oxford from 1957 to 1977...
at the University of Oxford.

Origin of an EPR signal
Every electron has a magnetic momentMagnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
and spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
quantum number
Quantum number
Quantum numbers describe values of conserved quantities in the dynamics of the quantum system. Perhaps the most peculiar aspect of quantum mechanics is the quantization of observable quantities. This is distinguished from classical mechanics where the values can range continuously...
s = 1/2, with magnetic components ms = +1/2 and ms = −1/2. In the presence of an external magnetic field with strength B0, the electron's magnetic moment aligns itself either parallel (ms = −1/2) or antiparallel (ms = +1/2) to the field, each alignment having a specific energy (see the Zeeman effect
Zeeman effect
The Zeeman effect is the splitting of a spectral line into several components in the presence of a static magnetic field. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field...
). The parallel alignment corresponds to the lower energy state, and the separation between it and the upper state is
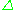 E = geμBB0, where ge is the electron's so-called g-factor (see also the Landé g-factor
E = geμBB0, where ge is the electron's so-called g-factor (see also the Landé g-factorLandé g-factor
In physics, the Landé g-factor is a particular example of a g-factor, namely for an electron with both spin and orbital angular momenta. It is named after Alfred Landé, who first described it in 1921....
) and μB is the Bohr magneton. This equation implies that the splitting of the energy levels is directly proportional to the magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
's strength, as shown in the diagram below.

 = h
= h such that the resonance condition,
such that the resonance condition,  =
= 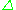 E, is obeyed. Substituting in
E, is obeyed. Substituting in  = h
= h and
and 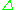 E = geμBB0 leads to the fundamental equation of EPR spectroscopy: h
E = geμBB0 leads to the fundamental equation of EPR spectroscopy: h = geμBB0. Experimentally, this equation permits a large combination of frequency and magnetic field values, but the great majority of EPR measurements are made with microwaves in the 9000–10000 MHz (9–10 GHz) region, with fields corresponding to about 3500 G (0.35 T). See below for other field-frequency combinations.
= geμBB0. Experimentally, this equation permits a large combination of frequency and magnetic field values, but the great majority of EPR measurements are made with microwaves in the 9000–10000 MHz (9–10 GHz) region, with fields corresponding to about 3500 G (0.35 T). See below for other field-frequency combinations.In principle, EPR spectra can be generated by either varying the photon frequency incident on a sample while holding the magnetic field constant or doing the reverse. In practice, it is usually the frequency that is kept fixed. A collection of paramagnetic centers, such as free radicals, is exposed to microwaves at a fixed frequency. By increasing an external magnetic field, the gap between the ms = +1/2 and ms = −1/2 energy states is widened until it matches the energy of the microwaves, as represented by the double-arrow in the diagram above. At this point the unpaired electrons can move between their two spin states. Since there typically are more electrons in the lower state, due to the Maxwell-Boltzmann distribution (see below), there is a net absorption of energy, and it is this absorption that is monitored and converted into a spectrum.
As an example of how
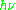 = geμBB0 can be used, consider the case of a free electron, which has ge = 2.0023, and the simulated spectrum shown at the right in two different forms. For the microwave frequency of 9388.2 MHz, the predicted resonance position is a magnetic field of about B0 = h
= geμBB0 can be used, consider the case of a free electron, which has ge = 2.0023, and the simulated spectrum shown at the right in two different forms. For the microwave frequency of 9388.2 MHz, the predicted resonance position is a magnetic field of about B0 = h / geμB = 0.3350 tesla = 3350 gauss, as shown. Note that while two forms of the same spectrum are presented in the figure, most EPR spectra are recorded and published only as first derivatives.
/ geμB = 0.3350 tesla = 3350 gauss, as shown. Note that while two forms of the same spectrum are presented in the figure, most EPR spectra are recorded and published only as first derivatives.Because of electron-nuclear mass differences, the magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
of an electron is substantially larger than the corresponding quantity for any nucleus, so that a much higher electromagnetic frequency is needed to bring about a spin resonance with an electron than with a nucleus, at identical magnetic field strengths. For example, for the field of 3350 G shown at the right, spin resonance occurs near 9388.2 MHz for an electron compared to only about 14.3 MHz for 1H nuclei. (For NMR spectroscopy, the corresponding resonance equation is
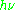 = gNμNB0 where gN and μN depend on the nucleus under study.)
= gNμNB0 where gN and μN depend on the nucleus under study.)Maxwell-Boltzmann distribution
In practice, EPR samples consist of collections of many paramagnetic species, and not single isolated paramagnetic centers. If the population of radicals is in thermodynamic equilibrium, its statistical distribution is described by the Maxwell-Boltzmann equation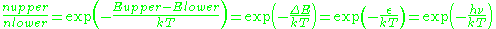
where
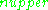 is the number of paramagnetic centers occupying the upper energy state,
is the number of paramagnetic centers occupying the upper energy state,  is the Boltzmann constant, and
is the Boltzmann constant, and  is the temperature in kelvin
is the temperature in kelvinKelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
s. At 298 K, X-band microwave frequencies (
 ≈ 9.75 GHz) give
≈ 9.75 GHz) give 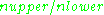 ≈ 0.998, meaning that the upper energy level has a smaller population than the lower one. Therefore, transitions from the lower to the higher level are more probable than the reverse, which is why there is a net absorption of energy.
≈ 0.998, meaning that the upper energy level has a smaller population than the lower one. Therefore, transitions from the lower to the higher level are more probable than the reverse, which is why there is a net absorption of energy.The sensitivity of the EPR method (i.e., the minimum number of detectable spins
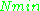 ) depends on the photon frequency
) depends on the photon frequency  according to
according to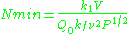
where
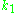 is a constant,
is a constant, 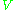 is the sample's volume,
is the sample's volume, 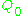 is the unloaded quality factor
is the unloaded quality factorQ factor
In physics and engineering the quality factor or Q factor is a dimensionless parameter that describes how under-damped an oscillator or resonator is, or equivalently, characterizes a resonator's bandwidth relative to its center frequency....
of the microwave cavity (sample chamber),
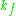 is the cavity filling coefficient, and
is the cavity filling coefficient, and 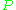 is the microwave power in the spectrometer cavity. With
is the microwave power in the spectrometer cavity. With 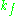 and
and 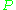 being constants,
being constants, 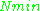 ~
~ 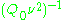 , i.e.,
, i.e., 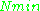 ~
~ 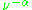 , where
, where  ≈ 1.5. In practice,
≈ 1.5. In practice,  can change varying from 0.5 to 4.5 depending on spectrometer characteristics, resonance conditions, and sample size. In other words, the higher the spectrometer frequency the lower the detection limit (
can change varying from 0.5 to 4.5 depending on spectrometer characteristics, resonance conditions, and sample size. In other words, the higher the spectrometer frequency the lower the detection limit (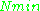 ), meaning greater sensitivity.
), meaning greater sensitivity.Spectral parameters
In real systems, electrons are normally not solitary, but are associated with one or more atoms. There are several important consequences of this:- An unpaired electron can gain or lose angular momentum, which can change the value of its g-factor, causing it to differ from ge. This is especially significant for chemical systems with transition-metal ions.
- If an atom with which an unpaired electron is associated has a non-zero nuclear spin, then its magnetic moment will affect the electron. This leads to the phenomenon of hyperfine coupling, analogous to J-couplingJ-couplingJ-coupling is the coupling between two nuclear spins due to the influence of bonding electrons on the magnetic field running between the two nuclei. J-coupling contains information about dihedral angles, which can be estimated using the Karplus equation...
in NMR, splitting the EPR resonance signal into doublets, triplets and so forth. - Interactions of an unpaired electron with its environment influence the shape of an EPR spectral line. Line shapes can yield information about, for example, rates of chemical reactions.
- The g-factor and hyperfine coupling in an atom or molecule may not be the same for all orientations of an unpaired electron in an external magnetic field. This anisotropyAnisotropyAnisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties An example of anisotropy is the light...
depends upon the electronic structure of the atom or molecule (e.g., free radical) in question, and so can provide information about the atomic or molecular orbital containing the unpaired electron.
The g factor
Knowledge of the g-factorLandé g-factor
In physics, the Landé g-factor is a particular example of a g-factor, namely for an electron with both spin and orbital angular momenta. It is named after Alfred Landé, who first described it in 1921....
can give information about a paramagnetic center's electronic structure. An unpaired electron responds not only to a spectrometer's applied magnetic field B0 but also to any local magnetic fields of atoms or molecules. The effective field Beff experienced by an electron is thus written
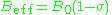
where
 includes the effects of local fields (
includes the effects of local fields ( can be positive or negative). Therefore, the h
can be positive or negative). Therefore, the h = geμBBeff resonance condition (above) is rewritten as follows:
= geμBBeff resonance condition (above) is rewritten as follows: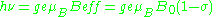
The quantity ge(1 – σ) is denoted g and called simply the g-factor, so that the final resonance equation becomes
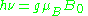
This last equation is used to determine g in an EPR experiment by measuring the field and the frequency at which resonance occurs. If g does not equal ge the implication is that the ratio of the unpaired electron's spin magnetic moment to its angular momentum differs from the free electron value. Since an electron's spin magnetic moment is constant (approximately the Bohr magneton), then the electron must have gained or lost angular momentum through spin-orbit coupling. Because the mechanisms of spin-orbit coupling are well understood, the magnitude of the change gives information about the nature of the atomic or molecular orbital containing the unpaired electron.
In general, the g factor is not a number
Scalar (mathematics)
In linear algebra, real numbers are called scalars and relate to vectors in a vector space through the operation of scalar multiplication, in which a vector can be multiplied by a number to produce another vector....
but a second-rank tensor
Tensor
Tensors are geometric objects that describe linear relations between vectors, scalars, and other tensors. Elementary examples include the dot product, the cross product, and linear maps. Vectors and scalars themselves are also tensors. A tensor can be represented as a multi-dimensional array of...
represented by nine numbers arranged in a 3×3 matrix
Matrix (mathematics)
In mathematics, a matrix is a rectangular array of numbers, symbols, or expressions. The individual items in a matrix are called its elements or entries. An example of a matrix with six elements isMatrices of the same size can be added or subtracted element by element...
. The principal axes
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
of this tensor are determined by the local fields, for example, by the local atomic arrangement around the unpaired spin in a solid or in a molecule. Choosing an appropriate coordinate system (say, x,y,z) allows to "diagonalize" this tensor thereby reducing the maximum number of its components from nine to three, gxx, gyy and gzz. For a single spin experiencing only Zeeman interaction with an external magnetic field, the position of the EPR resonance is given by the expression gxxBx + gyyBy + gzzBz. Here Bx, By and Bz are the components of the magnetic field vector in the coordinate system (x,y,z); their magnitudes change as the field is rotated, so as the frequency of the resonance. For a large ensemble of randomly oriented spins, the EPR spectrum consists three peaks of characteristic shape at frequencies gxxB0, gyyB0 and gzzB0: the low-frequency peak is positive in first-derivative spectra, the high-frequency peak is negative, and the central peak is bipolar. Such situation is commonly observed in powders and the spectra are therefore called "powder-pattern spectra". In crystals, the number of EPR lines is determined by the number of crystallographically equivalent orientations of the EPR spin (called "EPR center").
Hyperfine coupling
Since the source of an EPR spectrum is a change in an electron's spin state, it might be thought that all EPR spectra for a single electron spin would consist of one line. However, the interaction of an unpaired electron, by way of its magnetic moment, with nearby nuclear spins, results in additional allowed energy states and, in turn, multi-lined spectra. In such cases, the spacing between the EPR spectral lines indicates the degree of interaction between the unpaired electron and the perturbing nuclei. The hyperfine coupling constant of a nucleus is directly related to the spectral line spacing and, in the simplest cases, is essentially the spacing itself.Two common mechanisms by which electrons and nuclei interact are the Fermi contact interaction
Fermi contact interaction
The Fermi contact interaction is the magnetic interaction between an electron and an atomic nucleus when the electron is inside that nucleus. It is of magnitude...
and by dipolar interaction. The former applies largely to the case of isotropic interactions (independent of sample orientation in a magnetic field) and the latter to the case of anisotropic interactions (spectra dependent on sample orientation in a magnetic field). Spin polarization is a third mechanism for interactions between an unpaired electron and a nuclear spin, being especially important for
 -electron organic radicals, such as the benzene radical anion. The symbols "a" or "A" are used for isotropic hyperfine coupling constants while "B" is usually employed for anisotropic hyperfine coupling constants.
-electron organic radicals, such as the benzene radical anion. The symbols "a" or "A" are used for isotropic hyperfine coupling constants while "B" is usually employed for anisotropic hyperfine coupling constants.In many cases, the isotropic hyperfine splitting pattern for a radical freely tumbling in a solution (isotropic system) can be predicted.
- For a radical having M equivalent nuclei, each with a spin of I, the number of EPR lines expected is 2MI + 1. As an example, the methyl radical, CH3, has three 1H nuclei each with I = 1/2, and so the number of lines expected is 2MI + 1 = 2(3)(1/2) + 1 = 4, which is as observed.
- For a radical having M1 equivalent nuclei, each with a spin of I1, and a group of M2 equivalent nuclei, each with a spin of I2, the number of lines expected is (2M1I1 + 1) (2M2I2 + 1). As an example, the methoxymethyl radical, H2C(OCH3), has two equivalent 1H nuclei each with I = 1/2 and three equivalent 1H nuclei each with I = 1/2, and so the number of lines expected is (2M1I1 + 1) (2M2I2 + 1) = [2(2)(1/2) + 1][2(3)(1/2) + 1] = [3][4] = 12, again as observed.
- The above can be extended to predict the number of lines for any number of nuclei.
While it is easy to predict the number of lines a radical's EPR spectrum should show, the reverse problem, unraveling a complex multi-line EPR spectrum and assigning the various spacings to specific nuclei, is more difficult.
In the oft-encountered case of I = 1/2 nuclei (e.g., 1H, 19F, 31P), the line intensities produced by a population of radicals, each possessing M equivalent nuclei, will follow Pascal's triangle
Pascal's triangle
In mathematics, Pascal's triangle is a triangular array of the binomial coefficients in a triangle. It is named after the French mathematician, Blaise Pascal...
. For example, the spectrum at the right shows that the three 1H nuclei of the CH3 radical give rise to 2MI + 1 = 2(3)(1/2) + 1 = 4 lines with a 1:3:3:1 ratio. The line spacing gives a hyperfine coupling constant of aH = 23 G for each of the three 1H nuclei. Note again that the lines in this spectrum are first derivatives of absorptions.
As a second example, consider the methoxymethyl radical, H2C(OCH3). The two equivalent methyl hydrogens will give an overall 1:2:1 EPR pattern, each component of which is further split by the three methoxy hydrogens into a 1:3:3:1 pattern to give a total of 3×4 = 12 lines, a triplet of quartets. A simulation of the observed EPR spectrum is shown at the right, and agrees with the 12-line prediction and the expected line intensities. Note that the smaller coupling constant (smaller line spacing) is due to the three methoxy hydrogens, while the larger coupling constant (line spacing) is from the two hydrogens bonded directly to the carbon atom bearing the unpaired electron. It is often the case that coupling constants decrease in size with distance from a radical's unpaired electron, but there are some notable exceptions, such as the ethyl radical (CH2CH3).
Resonance linewidth definition
Resonance linewidths are defined in terms of the magnetic induction B, and its corresponding units, and are measured along the x axis of an EPR spectrum, from a line's center to a chosen reference point of the line. These defined widths are called halfwidths and possess some advantages: for asymmetric lines values of left and right halfwidth can be given. The halfwidth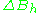 is the distance measured from the line's center to the point in which absorption
is the distance measured from the line's center to the point in which absorptionAbsorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
value has half of maximal absorption value in the center of resonance
Resonance
In physics, resonance is the tendency of a system to oscillate at a greater amplitude at some frequencies than at others. These are known as the system's resonant frequencies...
line. First inclination width
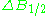 is a distance from center of the line to the point of maximal absorption curve inclination. In practice, a full definition of linewidth is used. For symmetric lines, halfwidth
is a distance from center of the line to the point of maximal absorption curve inclination. In practice, a full definition of linewidth is used. For symmetric lines, halfwidth 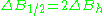 , and full inclination width
, and full inclination width 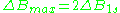
Pulsed EPR
The dynamics of electron spins are best studied with pulsed measurements . Microwave pulses typically 10–100 ns long are used to control the spins in the Bloch sphereBloch sphere
In quantum mechanics, the Bloch sphere is a geometrical representation of the pure state space of a two-level quantum mechanical system , named after the physicist Felix Bloch....
. The spin-lattice
Spin-lattice relaxation time
Spin–lattice relaxation is the mechanism by which the z component of the magnetization vector comes into thermodynamic equilibrium with its surroundings in nuclear magnetic resonance and magnetic resonance imaging. It is characterized by the spin–lattice relaxation time, a time constant known as T1...
relaxation time can be measured with an inversion recovery experiment.
As with pulsed NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
, the Hahn echo is central to many pulsed EPR experiments. A Hahn echo
Spin echo
In magnetic resonance, a spin echo is the refocusing of precessing spin magnetisation by a pulse of resonant radiation. Modern nuclear magnetic resonance and magnetic resonance imaging rely heavily on this effect....
decay experiment can be used to measure the dephasing time, as shown in the animation below. The size of the echo is recorded for different spacings of the two pulses. This reveals the decoherence, which is not refocused by the
 pulse. In simple cases, an exponential decay is measured, which is described by the
pulse. In simple cases, an exponential decay is measured, which is described by the 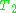 time.
time.Applications
EPR spectroscopy is used in various branches of science, such as chemistryChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, for the detection and identification of free radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s and paramagnetic centers such as F centers
F-Center
An F-Center or Farbe center is a type of crystallographic defect in which an anionic vacancy in a crystal is filled by one or more electrons, depending on the charge of the missing ion in the crystal. Electrons in such a vacancy tend to absorb light in the visible spectrum such that a material...
. EPR is a sensitive, specific method for studying both radicals formed in chemical reactions and the reactions themselves. For example, when frozen water (solid H2O) is decomposed by exposure to high-energy radiation, radicals such as H, OH, and HO2 are produced. Such radicals can be identified and studied by EPR. Organic and inorganic radicals can be detected in electrochemical systems and in materials exposed to UV light. In many cases, the reactions to make the radicals and the subsequent reactions of the radicals are of interest, while in other cases EPR is used to provide information on a radical's geometry and the orbital of the unpaired electron.
Medical
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
and biological
Biology
Biology is a natural science concerned with the study of life and living organisms, including their structure, function, growth, origin, evolution, distribution, and taxonomy. Biology is a vast subject containing many subdivisions, topics, and disciplines...
applications of EPR also exist. Although radicals are very reactive, and so do not normally occur in high concentrations in biology, special reagents have been developed to spin-label molecules of interest. These reagents are particularly useful in biological systems. Specially-designed nonreactive radical molecules can attach to specific sites in a biological cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
, and EPR spectra can then give information on the environment of these so-called spin-label
Spin label
A spin label is an organic molecule which possesses an unpaired electron, usually on a nitrogen atom, and the ability to bind to another molecule. Spin labels are normally used as tools for probing proteins or biological membrane-local dynamics using EPR spectroscopy. The site-directed spin...
or spin-probes
Spin probe
A spin probe is a molecule with stable free radical character that carries a functional group. This group can be used to couple the probe to another molecule, e.g. a biomolecule.Electron spin resonance can be employed to quantify the probe's concentration....
.
A type of dosimetry system
Dosimetry
Radiation dosimetry is the measurement and calculation of the absorbed dose in matter and tissue resulting from the exposure to indirect and direct ionizing radiation...
has been designed for reference standards and routine use in medicine, based on EPR signals of radicals from irradiated polycrystalline α-alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
(the alanine deamination radical, the hydrogen abstraction radical, and the (CO-(OH))=C(CH3)NH2+ radical) . This method is suitable for measuring gamma
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
and x-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s, electrons, protons, and high-linear energy transfer
Linear energy transfer
Linear energy transfer is a measure of the energy transferred to material as an ionizing particle travels through it. Typically, this measure is used to quantify the effects of ionizing radiation on biological specimens or electronic devices....
(LET) radiation of doses
Absorbed dose
Absorbed dose is a measure of the energy deposited in a medium by ionizing radiation per unit mass...
in the 1 Gy
Gray (unit)
The gray is the SI unit of absorbed radiation dose of ionizing radiation , and is defined as the absorption of one joule of ionizing radiation by one kilogram of matter ....
to 100 kGy range.
EPR spectroscopy can be applied only to systems in which the balance between radical decay and radical formation keeps the free-radicals concentration above the detection limit of the spectrometer used. This can be a particularly severe problem in studying reactions in liquids. An alternative approach is to slow down reactions by studying samples held at cryogenic temperatures, such as 77 K (liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
) or 4.2 K (liquid helium). An example of this work is the study of radical reactions in single crystals of amino acids exposed to x-rays, work that sometimes leads to activation energies
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
and rate constants for radical reactions.
The study of radiation-induced free radicals in biological substances (for cancer research) poses the additional problem that tissue contains water, and water (due to its electric dipole moment
Electric dipole moment
In physics, the electric dipole moment is a measure of the separation of positive and negative electrical charges in a system of charges, that is, a measure of the charge system's overall polarity with SI units of Coulomb-meter...
) has a strong absorption band in the microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
region used in EPR spectrometers.
EPR also has been used by archaeologists for the dating of teeth. Radiation damage over long periods of time creates free radicals in tooth enamel, which can then be examined by EPR and, after proper calibration, dated. Alternatively, material extracted from the teeth of people during dental procedures can be used to quantify their cumulative exposure to ionizing radiation. People exposed to radiation from the Chernobyl disaster
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
have been examined by this method.
Radiation-sterilized foods have been examined with EPR spectroscopy, the aim being to develop methods to determine if a particular food sample has been irradiated and to what dose.
Because of its high sensitivity, EPR was used recently to measure the quantity of energy used locally during a mechanochemical milling process.
EPR spectroscopy has been used to measure properties of crude oil, in particular asphaltene and vanadium
Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature...
content. EPR measurement of asphaltene content is a function of spin density and solvent polarity. Prior work dating to the 1960s has demonstrated the ability to measure vanadium
Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature...
content to sub-ppm levels.
High-field high-frequency measurements
High-field high-frequency EPR measurements are sometimes needed to detect subtle spectroscopic details. However, for many years the use of electromagnets to produce the needed fields above 1.5 T was impossible, due principally to limitations of traditional magnet materials. The first multifunctional millimeter EPR spectrometer with a superconducting solenoid was described in the early 1970s by Prof. Y. S. Lebedev's group (Russian Institute of Chemical Physics, MoscowMoscow
Moscow is the capital, the most populous city, and the most populous federal subject of Russia. The city is a major political, economic, cultural, scientific, religious, financial, educational, and transportation centre of Russia and the continent...
) in collaboration with L. G. Oranski's group (Ukrainian Physics and Technics Institute, Donetsk), which began working in the Institute of Problems of Chemical Physics
Institute of Problems of Chemical Physics
The Institute of Problems of Chemical Physics of the Russian Academy of Sciences is the largest Institute of the research center in Chernogolovka. The Institute consists of 10 scientific departments, about 100 laboratories and independent research groups. The staff of the Institute counts ca...
, Chernogolovka
Chernogolovka
Chernogolovka is a town in Moscow Oblast, Russia, located northeast from Moscow border. Population: Chernogolovka does not have a rail link but long distance buses link the town to Moscow, Noginsk and Fryanovo.-Research facilities:...
around 1975. Two decades later, a W-band EPR spectrometer was produced as a small commercial line by the German Bruker Company
Bruker
Bruker is a leading provider of high-performance scientific instruments and solutions for molecular and materials research, as well as for industrial and applied analysis...
, initiating the expansion of W-band EPR techniques into medium-sized academic laboratories. Today there still are only a few scientific centers in the world capable of high-field high-frequency EPR; among them are the Grenoble High Magnetic Field Laboratory in Grenoble
Grenoble
Grenoble is a city in southeastern France, at the foot of the French Alps where the river Drac joins the Isère. Located in the Rhône-Alpes region, Grenoble is the capital of the department of Isère...
, France
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
, the Physics Department in Freie Universität Berlin, the National High Magnetic Field Laboratory in Tallahassee, US, the National Center for Advanced ESR Technology (ACERT) at Cornell University
Cornell University
Cornell University is an Ivy League university located in Ithaca, New York, United States. It is a private land-grant university, receiving annual funding from the State of New York for certain educational missions...
in Ithaca
Ithaca
Ithaca or Ithaka is an island located in the Ionian Sea, in Greece, with an area of and a little more than three thousand inhabitants. It is also a separate regional unit of the Ionian Islands region, and the only municipality of the regional unit. It lies off the northeast coast of Kefalonia and...
, US, the Department of Physiology, and Biophysics at Albert Einstein College of Medicine
Albert Einstein College of Medicine
Albert Einstein College of Medicine is a graduate school of Yeshiva University. It is a not-for-profit, private, nonsectarian medical school located on the Jack and Pearl Resnick Campus in the Morris Park neighborhood of the borough of the Bronx of New York City...
, Bronx, NY, the IFW in Dresden
Dresden
Dresden is the capital city of the Free State of Saxony in Germany. It is situated in a valley on the River Elbe, near the Czech border. The Dresden conurbation is part of the Saxon Triangle metropolitan area....
, Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
, the Institute of Physics of Complex Matter in Lausanne
Lausanne
Lausanne is a city in Romandy, the French-speaking part of Switzerland, and is the capital of the canton of Vaud. The seat of the district of Lausanne, the city is situated on the shores of Lake Geneva . It faces the French town of Évian-les-Bains, with the Jura mountains to its north-west...
in Switzerland
Switzerland
Switzerland name of one of the Swiss cantons. ; ; ; or ), in its full name the Swiss Confederation , is a federal republic consisting of 26 cantons, with Bern as the seat of the federal authorities. The country is situated in Western Europe,Or Central Europe depending on the definition....
, and the Institute of Physics of the Leiden University
Leiden University
Leiden University , located in the city of Leiden, is the oldest university in the Netherlands. The university was founded in 1575 by William, Prince of Orange, leader of the Dutch Revolt in the Eighty Years' War. The royal Dutch House of Orange-Nassau and Leiden University still have a close...
, Netherlands
Netherlands
The Netherlands is a constituent country of the Kingdom of the Netherlands, located mainly in North-West Europe and with several islands in the Caribbean. Mainland Netherlands borders the North Sea to the north and west, Belgium to the south, and Germany to the east, and shares maritime borders...
.
| Waveband | L | S | C | X | P | K | Q | U | V | E | W | F | D | — | J | — |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
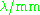 |
300 | 100 | 75 | 30 | 20 | 12.5 | 8.5 | 6 | 4.6 | 4 | 3.2 | 2.7 | 2.1 | 1.6 | 1.1 | 0.83 |
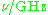 |
1 | 3 | 4 | 10 | 15 | 24 | 35 | 50 | 65 | 75 | 95 | 111 | 140 | 190 | 285 | 360 |
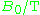 |
0.03 | 0.11 | 0.14 | 0.33 | 0.54 | 0.86 | 1.25 | 1.8 | 2.3 | 2.7 | 3.5 | 3.9 | 4.9 | 6.8 | 10.2 | 12.8 |
The EPR waveband is stipulated by the frequency or wavelength of a spectrometer's microwave source (see Table).
EPR experiments often are conducted at X
X band
The X band is a segment of the microwave radio region of the electromagnetic spectrum. In some cases, such as in communication engineering, the frequency range of X band is rather indefinitely set at approximately 7.0 to 11.2 gigahertz . In radar engineering, the frequency range is specified...
and, less commonly, Q bands, mainly due to the ready availability of the necessary microwave components (which originally were developed for radar applications). A second reason for widespread X and Q band measurements is that electromagnets can reliably generate fields up to about 1 tesla. However, the low spectral resolution over g-factor at these wavebands limits the study of paramagnetic centers with comparatively low anisotropic magnetic parameters. Measurements at
 > 40 GHz, in the millimeter wavelength region, offer the following advantages:
> 40 GHz, in the millimeter wavelength region, offer the following advantages:- EPR spectra are simplified due to the reduction of second-order effects at high fields.
- Increase in orientation selectivity and sensitivity in the investigation of disordered systems.
- The informativity and precision of pulse methodsPulse EPRPulsed electron paramagnetic resonance is a spectroscopic technique related to common nuclear magnetic resonance . Its most basic form involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short...
, e.g., ENDOREndorEndor or Ein Dor may refer to:* Endor , from the Hebrew Bible, a Canaanite village where the Witch of Endor lived* Indur, a Palestinian village depopulated during the 1948 Arab-Israeli war* Ein Dor, a Kibbutz in modern Israel...
also increase at high magnetic fields. - Accessibility of spin systems with larger zero-field splitting due to the larger microwave quantum energy h
 .
. - The higher spectral resolution over g-factor, which increases with irradiation frequency
 and external magnetic field B0. This is used to investigate the structure, polarity, and dynamics of radical microenvironments in spin-modified organic and biological systems through the spin labelSpin labelA spin label is an organic molecule which possesses an unpaired electron, usually on a nitrogen atom, and the ability to bind to another molecule. Spin labels are normally used as tools for probing proteins or biological membrane-local dynamics using EPR spectroscopy. The site-directed spin...
and external magnetic field B0. This is used to investigate the structure, polarity, and dynamics of radical microenvironments in spin-modified organic and biological systems through the spin labelSpin labelA spin label is an organic molecule which possesses an unpaired electron, usually on a nitrogen atom, and the ability to bind to another molecule. Spin labels are normally used as tools for probing proteins or biological membrane-local dynamics using EPR spectroscopy. The site-directed spin...
and probe method. The figure shows how spectral resolution improves with increasing frequency. - Saturation of paramagnetic centers occurs at a comparatively low microwave polarizing field B1, due to the exponential dependence of the number of excited spins on the radiation frequency
 . This effect can be successfully used to study the relaxation and dynamics of paramagnetic centers as well as of superslow motion in the systems under study.
. This effect can be successfully used to study the relaxation and dynamics of paramagnetic centers as well as of superslow motion in the systems under study. - The cross-relaxation of paramagnetic centers decreases dramatically at high magnetic fields, making it easier to obtain more-precise and more-complete information about the system under study.
See also
- Ferromagnetic resonanceFerromagnetic resonanceFerromagnetic resonance, or FMR, is a spectroscopic technique to probe the magnetization of ferromagnetic materials. It is a standard tool for probing spin waves and spin dynamics...
- Dynamic nuclear polarisationDynamic nuclear polarisationDynamic nuclear polarization results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann...
- Spin labelSpin labelA spin label is an organic molecule which possesses an unpaired electron, usually on a nitrogen atom, and the ability to bind to another molecule. Spin labels are normally used as tools for probing proteins or biological membrane-local dynamics using EPR spectroscopy. The site-directed spin...
s - Site-directed spin labelingSite-directed spin labelingSite-directed spin labeling is a technique for investigating protein local dynamics using electron spin resonance. The theory of SDSL is based on the specific reaction of spin labels with amino acids. A spin label's built-in protein structure can be detected by EPR spectroscopy...
- Spin trappingSpin trappingSpin trapping in chemistry is an analytical technique employed in the detection and identification of short-lived free radicals. Spin trapping involves the addition of radical to a nitrone spin trap resulting in the formation of a spin adduct, a nitroxide-based persistent radical, that can be...
External links
- Electron Magnetic Resonance Program National High Magnetic Field Laboratory
- Electron Paramagnetic Resonance (Specialist Periodical Reports) Published by the Royal Society of ChemistryRoyal Society of ChemistryThe Royal Society of Chemistry is a learned society in the United Kingdom with the goal of "advancing the chemical sciences." It was formed in 1980 from the merger of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society and the Society for Analytical Chemistry with a new...

