
Uranyl
Encyclopedia


Oxycation
An oxycation is a polyatomic ion with a positive charge that contains oxygen.-Examples:* Nitrosonium ion, NO+* Nitronium ion, NO2+* Vanadyl ion, VO2+, a very stable oxycationSee category for a bigger list....
of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
in the oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
+6, with the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
[UO2]2+. It has a linear structure with short U-O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s are bound to the uranyl ion in an equatorial plane. The uranyl ion forms many complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.
Structure and Bonding

Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
], the electrons used in forming the U-O bonds are supplied by the oxygen atoms. The electrons are donated into empty atomic orbitals on the uranium atom. The empty orbitals of lowest energy are 7s, 5f and 6d. In terms of valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
the sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
s may be formed using
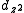 and
and 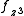 to construct sd, sf and df hybrid orbitals (the z axis passes through the oxygen atoms). (
to construct sd, sf and df hybrid orbitals (the z axis passes through the oxygen atoms). (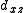 ,
, 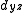 ) and (
) and (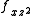 and
and  ) may be used to form pi bond
) may be used to form pi bondPi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
s. Since the pair of d or f orbitals used in bonding are doubly degenerate this equates to an overall bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
of three.
The uranyl ion is always associated with other ligands. The most common arrangement is for the so-called equatorial ligands to lie in a plane perpendicular to the O-U-O line and passing through the uranium atom. With four ligands, as in [UO2Cl4]2- the uranium has a distorted octahedral environment. In many cases there are more than four equatorial ligands. The presence of the equatorial ligands lowers the symmetry
Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
of the uranyl ion from point group D∞h for the isolated ion to, for example, D4h in a distorted octahedral complex; this permits the involvement of d and f orbitals other than those used in U-O bonds.
In uranyl fluoride
Uranyl fluoride
Uranyl fluoride , a compound of uranium, is an intermediate in the conversion of uranium hexafluoride UF6 to an uranium oxide or metal form and is a direct product of the reaction of UF6 with moisture in the air. It is very soluble in water. Uranyl fluoride also is hygroscopic and changes in color...
, UO2F2, the uranium atom achieves a coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
of 8 by forming a layer structure with two oxygen atoms in a uranyl configuration and six fluoride ions bridging between uranyl groups. A similar structure is found in α-uranium trioxide
Uranium trioxide
Uranium trioxide , also called uranyl oxide, uranium oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.-Production and use:There are three methods...
, with oxygen in place of fluoride, except that in that case the layers are connected by sharing oxygen atom from "uranyl groups", which are identified by having relatively short U-O distances. A similar structure occurs in some uranates, such as calcium uranate, CaUO4, which may be written as Ca(UO2)O2 even though the structure does not contain isolated uranyl groups.
Spectroscopy
The colour of uranyl compounds is due to LMCT charge transferCharge transfer complex
A charge-transfer complex or electron-donor-acceptor complex is an association of two or more molecules, or of different parts of one very large molecule, in which a fraction of electronic charge is transferred between the molecular entities. The resulting electrostatic attraction provides a...
transitions at ca. 420 nm, on the blue edge of the visible spectrum
Visible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm. In terms of...
. The exact location of the absorption band and NEXAFS bands depends on the nature of the equatorial ligands. Compounds containing the uranyl ion are usually yellow, though some compounds are red, orange or green.
Uranyl compounds also exhibit fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
. The first study of the green fluorescence of uranium glass
Uranium glass
Uranium glass is glass which has had uranium, usually in oxide diuranate form, added to a glass mix before melting. The proportion usually varies from trace levels to about 2% by weight uranium, although some 19th-century pieces were made with up to 25% uranium.Uranium glass was once made into...
, by Brewster
David Brewster
Sir David Brewster KH PRSE FRS FSA FSSA MICE was a Scottish physicist, mathematician, astronomer, inventor, writer and university principal.-Early life:...
in 1849, began extensive studies of the spectroscopy of the uranyl ion. Detailed understanding of this spectrum was obtained 130 years later. The fluorescence from K2UO2(SO4)2 was involved in the discovery of radioactivity.
The uranyl ion has characteristic ν U-O stretching vibrations
Molecular vibration
A molecular vibration occurs when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational and rotational motion...
at ca. 880 cm−1 (Raman spectrum) and 950 cm−1 (infrared spectrum). These frequencies depend somewhat on which ligands are present in the equatorial plane. Correlations are available between the stretching frequency and U-O bond length. It has also been observed that the stretching frequency correlates with the position of the equatorial ligands in the spectrochemical series
Spectrochemical series
A spectrochemical series is a list of ligands ordered on ligand strength and a list of metal ions based on oxidation number, group and its identity...
.
Aqueous chemistry
- [U(H2O)n ]6+ → [UO2(H2O)4]2+ + 4H+ + n-4 H2O
The driving force for this hypothetical reaction is the reduction in charge density on the uranium atom. The number of water molecules attached to the uranyl ion in aqueous solution is four. Further hydrolysis occurs, with a further reduction in charge density when one or more equatorial water molecules is replaced by an hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ion. In fact the aqueous uranyl ion is a weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
.
- [UO2(H2O)4]2+ [UO2(H2O)3(OH) ]+ + H+; pKaAcid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
= ca. 4.2
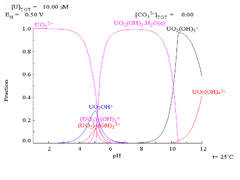
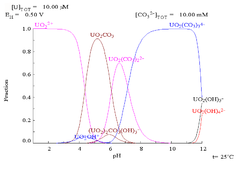
As pH increases polymeric species with stoichiometry [(UO2)2(OH)2]2+ and [(UO2)3(OH)5]+ are formed before the hydroxide UO2(OH)2 precipitates. The hydroxide dissolves in strongly alkaline solution to give hydroxo complexes of the uranyl ion.
The uranyl ion can be reduced by mild reducing agents, such as zinc metal, to the oxidation state +4. Reduction to uranium(3+) can be done using a Jones reductor
Jones reductor
A Jones reductor is a device which can be used to reduce a metal ion in aqueous solution to a very low oxidation state. The active component is a zinc/mercury amalgam...
.
Complexes
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
acceptor and forms weaker complexes with nitrogen-donor ligands than with fluoride and oxygen donor ligands, such as hydroxide, carbonate
Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
, nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
, sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
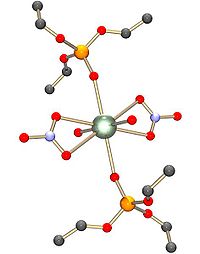
and carboxylate. There may be 4, 5 or 6 donor atoms in the equatorial plane. In uranyl nitrate, [UO2(NO3)2].2H2O, for example, there are six donor atoms in the equatorial plane, four from bidentate nitrato ligands and two from water molecules. The structure is described as hexagonal bipyramid
Hexagonal bipyramid
A hexagonal bipyramid is a polyhedron formed from two hexagonal pyramids joined at their bases. The resulting solid has 12 triangular faces, 8 vertices and 18 edges. The 12 faces are identical isosceles triangles.It is one of an infinite set of bipyramids...
al. Other oxygen-donor ligands include phosphine oxide
Phosphine oxide
Phosphine oxides are either inorganic phosphorus compounds such as phosphoryl trichloride or organophosphorus compounds with the formula OPR3, where R = alkyl or aryl...
s and phosphate esters.
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. The complex that is extracted has two nitrato ligands bound to the uranyl ion, making a complex with no electrical charge and also the water molecules are replaced by ether molecules, giving the whole complex notable hydrophobic character. Electroneutrality is the most imortant factor in making the complex soluble in organic solvents. The nitrate ion forms much stronger complexes with the uranyl ion than it does with transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
and lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
ions. For this reason only uranyl and other actinyl ions, including the plutonyl ion, PuO22+, can be extracted from mixtures containing other ions. Replacing the water molecules that are bound to the uranyl ion in aqueous solution by a second, hydrophobic, ligand increases the solubility of the neutral complex in the organic solvent. This has been called a synergic effect.
The complexes formed by the uranyl ion in aqueous solution are of major importance both in the extraction of uranium from its ores and in nuclear fuel reprocessing. In industrial processes uranyl nitrate is extracted with tributyl phosphate
Tributyl phosphate
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the formula 3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of orthophosphoric acid with n-butanol.- Production :Tributyl phosphate is manufactured by...
, (CH3CH2CH2CH2O)3PO, TBP, as the preferred second ligand, and kerosene the preferred organic solvent. Later in the process, uranium is stripped from the organic solvent by treating it with strong nitric acid, which forms complexes such as [UO2(NO3)4]2- which are more soluble in the aqueous phase. Uranyl nitrate is recovered by evaporating the solution.
Minerals
The uranyl ion occurs in minerals derived from uranium oreUraninite
Uraninite is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2, but also contains UO3 and oxides of lead, thorium, and rare earth elements...
deposits by water-rock interactions that occur in uranium-rich mineral seams. Tyuyamunite (Ca(UO2)2V2O8•8H2O), autunite
Autunite
Autunite with formula: Ca22·10-12H2O is a yellow - greenish fluorescent mineral with a hardness of 2 - 2½. Autunite crystallizes in the tetragonal system and often occurs as tabular square crystals. Due to the moderate uranium content of 48.27% it is radioactive and also used as uranium ore...
(Ca(UO2)2(PO4)2•8-12H2O), torbernite
Torbernite
The chemical formula of torbenite is similar to that of autunite in which a Cu2+ cation replaces a Ca2+. The number of water hydration molecules can vary between 12 and 8, giving rise to the variety of metatorbernite when torbernite spontaneously dehydrates...
(Cu(UO2)2 (PO4)•8-12H2O) and uranophane
Uranophane
Uranophane Ca22·5H2O is a rare calcium uranium silicate hydrate mineral that forms from the oxidation of uranium bearing minerals. Uranophane is also known as uranotile. It has a yellow color and is radioactive....
(H3O)2Ca (UO2)2(SiO4)•3H2O) are examples of uranyl containing minerals. These minerals are of little commercial value as most uranium is extracted from pitchblende.
Uses
Uranyl salts are used to stain samples for electron and electromagnetic microscopy studies of DNA.Health and environmental issues
Uranyl salts are toxic and can cause severe renal insufficiency and acute tubular necrosisAcute tubular necrosis
Acute tubular necrosis or is a medical condition involving the death of tubular cells that form the tubule that transports urine to the ureters while reabsorbing 99% of the water . Tubular cells continually replace themselves and if the cause of ATN is removed then recovery is likely...
. Target organs include the kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
s, liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
, lungs and brain
Brain
The brain is the center of the nervous system in all vertebrate and most invertebrate animals—only a few primitive invertebrates such as sponges, jellyfish, sea squirts and starfishes do not have one. It is located in the head, usually close to primary sensory apparatus such as vision, hearing,...
.
Uranyl ion accumulation in tissues including gonocytes produces congenital disorder
Congenital disorder
A congenital disorder, or congenital disease, is a condition existing at birth and often before birth, or that develops during the first month of life , regardless of causation...
s, and in white blood cells causes immune system damage. Uranyl compounds are also neurotoxin
Neurotoxin
A neurotoxin is a toxin that acts specifically on nerve cells , usually by interacting with membrane proteins such as ion channels. Some sources are more general, and define the effect of neurotoxins as occurring at nerve tissue...
s. Uranyl ion contamination has been found on and around depleted uranium
Depleted uranium
Depleted uranium is uranium with a lower content of the fissile isotope U-235 than natural uranium . Uses of DU take advantage of its very high density of 19.1 g/cm3...
targets.
All uranium compounds are radioactive. However, uranium is usually in depleted form, except in the context of the nuclear industry. Depleted uranium consists mainly of 238U
Isotopes of uranium
Uranium is a naturally occurring radioactive element that has no stable isotopes but two primordial isotopes that have long half-life and are found in appreciable quantity in the Earth's crust, along with the decay product uranium-234. The average atomic mass of natural uranium is 238.02891 u...
which decays by alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
with a half-life of 4.468(3)×109 y. Since it is a weak alpha emitter its radioactivity is only hazardous with direct contact or ingestion.

