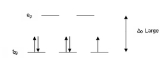
Crystal field theory
Overview
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
compounds, all of which can be considered coordination complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
. CFT successfully accounts for some magnetic properties, colours, hydration
Hydration reaction
In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
enthalpies
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
, and spinel
Spinel
Spinel is the magnesium aluminium member of the larger spinel group of minerals. It has the formula MgAl2O4. Balas ruby is an old name for a rose-tinted variety.-Spinel group:...
structures of transition metal complexes, but it does not attempt to describe bonding. CFT was developed by physicists Hans Bethe
Hans Bethe
Hans Albrecht Bethe was a German-American nuclear physicist, and Nobel laureate in physics for his work on the theory of stellar nucleosynthesis. A versatile theoretical physicist, Bethe also made important contributions to quantum electrodynamics, nuclear physics, solid-state physics and...
and John Hasbrouck van Vleck
John Hasbrouck van Vleck
John Hasbrouck Van Vleck was an American physicist and mathematician, co-awarded the 1977 Nobel Prize in Physics, for his contributions to the understanding of the behavior of electrons in magnetic solids....
in the 1930s. CFT was subsequently combined with molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
to form the more realistic and complex ligand field theory
Ligand field theory
Ligand field theory describes the bonding, orbital arrangement, and other characteristics of coordination complexes. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valence atomic orbitals, five d, one s, and three p orbitals...
(LFT), which delivers insight into the process of chemical bonding in transition metal complexes.
According to CFT, the interaction between a transition metal and ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s arises from the attraction between the positively charged metal cation and negative charge on the non-bonding electrons of the ligand.

