
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors
Encyclopedia
Discovery and development of nucleoside
and nucleotide
reverse-transcriptase inhibitors (NRTIs and NtRTIs) began in the 1980s when the AIDS
epidemic
hit Western societies. NRTIs inhibit the reverse transcriptase
(RT), an enzyme
that controls the replication of the genetic material of the human immunodeficiency virus (HIV
). The first NRTI was zidovudine
, approved by the U.S. Food and Drug Administration
(FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant
virus
es are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.
, zalcitabine, stavudine
, lamivudine
, abacavir
and emtricitabine
and the only nucleotide reverse-transcriptase inhibitor (NtRTI) approved is tenofovir (see table 4).
activity. It converts the viral single-stranded RNA
into an integration competent double stranded DNA
. Subsequently the generated DNA is translocated into the nucleus
of the host cell
where it is integrated in its genome
by the retroviral integrase. The other role of the RT is its ribonuclease H activity that degrades RNA only when it is in a heteroduplex
with DNA.
heterodimer which is 1000 amino acid
long and is composed of two subunit
s. The larger subunit, p66, is 560 amino acid long and it exhibits all the enzymatic activities of the RT.The smaller subunit, called p51, is 440 amino acid long and it is considered to stabilizes the heterodimer but also it may take part in the binding of the tRNA primer. The p66 subunit has the two active sites: polymerase and ribonuclease H. The polymerase has four subdomains that have been named “fingers“, “thumb“, “connection“ and “palm“ for it has been compared to the right hand.
NRTIs are analogues of endogenous
2´-deoxy-nucleoside and nucleotide. They are inactive in their parent forms and require successive phosphorylation
.
Nucleosides must be triphosphorylated, while nucleotides, which possess one phosphonated group, must be diphosphorylated. This stepwise activation process occurs inside the cell and is mediated by a coordinated series of enzymes. The first, and often rate limiting
, phosphorylation step (for nucleoside analogues) are most commonly catalyzed by deoxynucleoside kinases. Addition of the second phosphate group to nucleoside monophosphate analogues is completed by the nucleoside monophosphate kinases (NMP kinases). A variety of enzymes are able to catalyze the final phosphorylation step for NRTIs, including nucleoside diphosphate kinase (NDP kinase), phosphoglycerate kinase
, pyruvate kinase
and creatine kinase
, resulting in formation of respective antivirally active triphosphate analogues.
In their respective triphosphate forms, NRTIs and the only NtRTI available compete with their corresponding endogenous deoxynucleotide triphosphate (dNTPs) for incorporation into the nascent DNA chain (see figure 1). Unlike dNTPs substrate, NRTIs lack a 3´-hydroxyl group on the deoxyribose
moiety. Once incorporated into the DNA chain, the absence of a 3´-hydroxyl group, which normally forms the 5´- to 3´- phosphoester bond with the next nucleic acid
, blocks further extension of the DNA by RT, and they act as chain terminators.
. This compound had been prepared in 1964 as a potential anti-cancer agent but was shown to be ineffective. In 1974 zidovudine was reported to have activity against retroviruses and was subsequently re-screened as an antiviral when the AIDS epidemic hit Western societies during mid 1980‘s. However, ziduvodine is relatively toxic since it is converted into the triphosphate by the cellular enzymes and therefore it is activated in uninfected cells.
Dideoxynucleosides are analogues of nucleoside where the sugar ring lacks both 2´ and 3´-hydroxyl groups. Three years after the synthesis
of zidovudine, Jerome Horwitz and his colleagues in Chicago prepared another dideoxynucleoside now known as zalcitabine (ddC). Zalcitabine is a synthetic pyrimidine
nucleoside analogue, structurally related to deoxycytidine
, in which the 3´-hydroxyl group of the ribose
sugar moiety is substituted with hydrogen. Zalcitabine was approved by the FDA for the treatment of HIV-1 in June 1992.
2´,3´-dideoxyinosine or didanosine
is converted into dideoxyadenosine in vivo. Its development has a long history. In 1964 dideoxyadenosine, the corresponding adenosine analogue of zalcitabine was synthesised. Dideoxyadenosine caused kidney
damage so didanosine was prepared from dideoxyadenosine by enzymatic oxidation (see table 1). It was found to be active against HIV without causing kidney damage. Didanosine was approved by the FDA for the treatment of HIV-1 in October 1991.
Zalcitabine and didanosine are both obligate chain terminators, that have been developed for anti-HIV treatment. Unfortunately, both drugs lack selectivity
and therefore cause side-effects.
Further modification of the dideoxy framework led to the development of 2´,3´-didehydro-3´-deoxythymidine (stavudine, d4T). Activity of stavudine was shown to be similar to that of zidovudine, although their phosphorylation patterns differ; the
affinity
for zidovudine to thymidine kinase
(the enzyme responsible for the first phosphorylation) is similar to that of thymidine
, whereas the affinity for stavudine is 700-fold weaker.
2',3'‐dideoxy‐3'‐thiacytidine (lamivudine, 3TC) was discovered by Bernard Belleau
. The history of lamivudine can be traced back to the mid‐seventies while Bernard Belleau was investigating sugar derivative
s. Lamivudine was developed as the sulfur
analogue of zalcitabine (see table 2). It was initially synthesized as a racemic
mixture (BCH-189) and analysis showed that both positive and negative enantiomers of BCH-189 had in vitro activity against HIV. Lamivudine which is the negative enantiomer of 2',3'‐dideoxy‐3'‐thiacytidine and is a pyrimidine nucleoside analogue. The 3' carbon of the ribose ring of 2'-deoxycytidine has been replaced by a sulfur atom because it had greater anti-HIV activity and is less toxic than the positive enantiomer.
Next in line was 2',3'‐dideoxy‐5-fluoro-3'‐thiacytidine (Emtricitabine, FTC) which is
a structural homologue
of lamivudine. The structural difference is the 5-fluoro-modification of the base moiety of lamivudine. It is similar in many ways to lamivudine and is active against both HIV-1 and hepatitis B virus (HBV
).
use. That was 2´,3´-didehydro analogue of dideoxyadenosine. Insertion of a cyclopropyl group on its 6-amino nitrogen
of the adenine
ring increased lipophilicity and thus enhanced brain penetration. The resulting compound is known as abacavir (see table 3). Abacavir was approved by the FDA for use in therapy of HIV-1 infections in December 1998.
This drug is the only approved antiretroviral that is active as a guanosine
analogue in vivo. First it is monophosphorylated by adenosine phosphotransferase and then the monophosphate is converted to carbovir 3´-monophosphate. Subsequently it is fully phosphorylated and the carbovir is incorporated by the RT into the DNA chain and acts as a chain terminator. Carbovir is a related guanosine analogue that had poor oral bioavailability
and thus was withdrawn from clinical development.
in the phosphorylation requirement may allow more rapid and complete conversion of drugs to their active metabolites. Such considerations have led to the development of phosphonate nucleotide analogues such as tenofovir. Tenofovir disoproxil fumarate (Tenofovir DF) is the prodrug
of tenofovir. Tenofovir is an acyclic adenosine derivative. The acyclic nature of the compound and its phosphonate moiety are unique structural features among the approved NRTIs. Tenofovir DF is hydrolyzed enzymatically to tenofovir which exhibits anti-HIV activity. It was developed by the synthesis and broad spectrum antiviral activity of 2,3-dihydroxypropyladenine. Tenofovir DF was the first nucleotide reverse-transcriptase inhibitor approved by the FDA for the treatment of HIV-1 infection in October 2001.
Two main mechanisms are known that cause NRTI drug resistance: Interference with the incorporation of NRTIs and excision of incorporated NRTIs.Interference with the incorporated NRTIs involves a mutation
in the p66 subdomain of the RT.The mutation causes a steric hindrance that can exclude certain drugs, for example lamivudine, from being incorporated during reverse transcription. In case of excision of incorporated NRTIs the resistant enzymes readily accept the inhibitor as a substrate for incorporation into the DNA chain. Subsequently the RT enzyme can remove the incorporated NRTI by reversing the polymerization
step. The excision reaction requires a pyrophosphate donor which RT joins to the NRTI at the 3´primer terminus, excising it from the primer DNA.
To achieve efficient inhibition of HIV-1 replication in patients, and to delay or prevent appearance of drug resistant viruses, drug combinations are used. HAART, also known as highly active antiretroviral therapy consists of combinations of antiviral drugs which include NRTIs, NtRTI, non-nucleoside reverse-transcriptase inhibitors and protease inhibitors.
and the sulfur are essentially reversed. Even though apricitabine is a little less potent in vitro compared to some other NRTIs, it maintains its activity against a broad spectrum of HIV-1 variants with NRTI resistance mutations. Apricitabine is in the final stage of clinical development for the treatment of NRTI-experienced patients.
is a deoxycytidine analogue with activity against HIV resistant to several other nucleoside analogues, including zidovudine and lamivudine. This is partly because of high intracellular
levels of its triphosphate metabolite
reached in cells. Clinical trials of elvucitabine are on hold, because it has shown bone marrow suppression
in some patients, with CD4+ cell numbers dropping as early as two days after initiation of dosing.
is a guanosine analogue NRTI prodrug that has good bioavailability.It is deaminated intracellularly by adenosine deaminase
to dioxolane
guanine (DXG). DXG-triphosphate, the active form of the drug, has greater activity than DAPD-triphosphate. Amdoxovir is currently in phasa II clinical trials.
is a racemic mixture of the two β-enantiomers of emtricitabine (FTC), (-)-FTC and (+)-FTC. Racivir has excellent oral bioavailability and has the advantage of needing to be taken only once a day. Racivir can be considered to be used in combination of two NRTIs and has shown promising antiviral activity when used in combination. Racivir is currently in phase II clinical trials.
There are several more NRTIs in development. Either the sponsors have filed for an Investigational New Drug
(IND) application, the application has been approved by the FDA or the drugs are in different phases of clinical trails. Some of the NRTIs that are in development exhibit various attractive pharmacological properties that could make them desirable for the treatment of patients in need of new agents.
Nucleoside
Nucleosides are glycosylamines consisting of a nucleobase bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage...
and nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
reverse-transcriptase inhibitors (NRTIs and NtRTIs) began in the 1980s when the AIDS
AIDS
Acquired immune deficiency syndrome or acquired immunodeficiency syndrome is a disease of the human immune system caused by the human immunodeficiency virus...
epidemic
Epidemic
In epidemiology, an epidemic , occurs when new cases of a certain disease, in a given human population, and during a given period, substantially exceed what is expected based on recent experience...
hit Western societies. NRTIs inhibit the reverse transcriptase
Reverse transcriptase
In the fields of molecular biology and biochemistry, a reverse transcriptase, also known as RNA-dependent DNA polymerase, is a DNA polymerase enzyme that transcribes single-stranded RNA into single-stranded DNA. It also helps in the formation of a double helix DNA once the RNA has been reverse...
(RT), an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that controls the replication of the genetic material of the human immunodeficiency virus (HIV
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
). The first NRTI was zidovudine
Zidovudine
Zidovudine or azidothymidine is a nucleoside analog reverse-transcriptase inhibitor , a type of antiretroviral drug used for the treatment of HIV/AIDS. It is an analog of thymidine....
, approved by the U.S. Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
(FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant
Drug resistance
Drug resistance is the reduction in effectiveness of a drug such as an antimicrobial or an antineoplastic in curing a disease or condition. When the drug is not intended to kill or inhibit a pathogen, then the term is equivalent to dosage failure or drug tolerance. More commonly, the term is used...
virus
Virus
A virus is a small infectious agent that can replicate only inside the living cells of organisms. Viruses infect all types of organisms, from animals and plants to bacteria and archaea...
es are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.
History
In the summer of 1981 the acquired immunodeficiency syndrome (AIDS) was first reported. Two years later the etiological link to AIDS, the human immunodeficiency virus (HIV) was identified. Since the identification of HIV the development of effective antiretroviral drugs and the scientific achievements in HIV research has been enormous. Antiretroviral drugs for the treatment of HIV infections belong to six categories: Nucleoside and nucleotide reverse-transcriptase inhibitors, Non-nucleoside reverse-transcriptase inhibitors, protease inhibitors, entry inhibitors, co-receptor inhibitors and integrase inhibitors. The reverse transcriptase of HIV-1 has been the main foundation for the development of anti-HIV drugs. The first nucleoside reverse-transcriptase inhibitor with in vitro anti-HIV activity was zidovudine. Since zidovudine was approved in 1987, six nucleosides and one nucleotide reverse-transcriptase inhibitor (NRTI) have been approved by FDA. NRTIs approved by the FDA are zidovudine, didanosineDidanosine
Didanosine is sold under the trade names Videx and Videx EC. It is a reverse transcriptase inhibitor, effective against HIV and used in combination with other antiretroviral drug therapy as part of highly active antiretroviral therapy .-History:The related pro-drug of didanosine,...
, zalcitabine, stavudine
Stavudine
Stavudine is a nucleoside analog reverse transcriptase inhibitor active against HIV.-History:...
, lamivudine
Lamivudine
Lamivudine is a potent nucleoside analog reverse transcriptase inhibitor .It is marketed by GlaxoSmithKline with the brand names Zeffix, Heptovir, Epivir, and Epivir-HBV.Lamivudine has been used for treatment of chronic hepatitis B at a lower dose than for treatment of HIV...
, abacavir
Abacavir
Abacavir is a nucleoside analog reverse transcriptase inhibitor used to treat HIV and AIDS. It is available under the trade name Ziagen and in the combination formulations Trizivir and Kivexa/Epzicom...
and emtricitabine
Emtricitabine
Emtricitabine , with trade name Emtriva , is a nucleoside reverse transcriptase inhibitor for the treatment of HIV infection in adults and children....
and the only nucleotide reverse-transcriptase inhibitor (NtRTI) approved is tenofovir (see table 4).
Function
Most standard HIV drug thearapies revolve around inhibiting the reverse transcriptase enzyme (RT), an enzyme that is necessary to the HIV-1 virus and other retroviruses to complete their life cycle. The RT enzyme serves two key functions. First, it controls the replication of the viruses genetic material via its polymerasePolymerase
A polymerase is an enzyme whose central function is associated with polymers of nucleic acids such as RNA and DNA.The primary function of a polymerase is the polymerization of new DNA or RNA against an existing DNA or RNA template in the processes of replication and transcription...
activity. It converts the viral single-stranded RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
into an integration competent double stranded DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. Subsequently the generated DNA is translocated into the nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
of the host cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
where it is integrated in its genome
Genome
In modern molecular biology and genetics, the genome is the entirety of an organism's hereditary information. It is encoded either in DNA or, for many types of virus, in RNA. The genome includes both the genes and the non-coding sequences of the DNA/RNA....
by the retroviral integrase. The other role of the RT is its ribonuclease H activity that degrades RNA only when it is in a heteroduplex
Heteroduplex
A heteroduplex is a double-stranded molecule of nucleic acid originated through the genetic recombination of single complementary strands derived from different sources, such as from different homologous chromosomes or even from different organisms....
with DNA.
Structure
HIV-1 RT is an asymmetricAsymmetric
Something which is asymmetric displays asymmetry. Specific uses of the term may include:*Asymmetric relation for information on such relations in mathematics and set theory*Asymmetric warfare for information and theories of modern war...
heterodimer which is 1000 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
long and is composed of two subunit
Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
s. The larger subunit, p66, is 560 amino acid long and it exhibits all the enzymatic activities of the RT.The smaller subunit, called p51, is 440 amino acid long and it is considered to stabilizes the heterodimer but also it may take part in the binding of the tRNA primer. The p66 subunit has the two active sites: polymerase and ribonuclease H. The polymerase has four subdomains that have been named “fingers“, “thumb“, “connection“ and “palm“ for it has been compared to the right hand.
Mechanism of action
Activation of nucleoside and nucleotide reverse-transcriptase inhibitors is primarily dependent on cellular entry by passive diffusion or carrier-mediated transport. NRTIs are highly hydrophilic and have limited membrane permeability and therefore this step is very important.NRTIs are analogues of endogenous
Endogenous
Endogenous substances are those that originate from within an organism, tissue, or cell. Endogenous retroviruses are caused by ancient infections of germ cells in humans, mammals and other vertebrates...
2´-deoxy-nucleoside and nucleotide. They are inactive in their parent forms and require successive phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
.
Nucleosides must be triphosphorylated, while nucleotides, which possess one phosphonated group, must be diphosphorylated. This stepwise activation process occurs inside the cell and is mediated by a coordinated series of enzymes. The first, and often rate limiting
Rate limiting
In computer networks, rate limiting is used to control the rate of traffic sent or received on a network interface. Traffic that is less than or equal to the specified rate is sent, whereas traffic that exceeds the rate is dropped or delayed...
, phosphorylation step (for nucleoside analogues) are most commonly catalyzed by deoxynucleoside kinases. Addition of the second phosphate group to nucleoside monophosphate analogues is completed by the nucleoside monophosphate kinases (NMP kinases). A variety of enzymes are able to catalyze the final phosphorylation step for NRTIs, including nucleoside diphosphate kinase (NDP kinase), phosphoglycerate kinase
Phosphoglycerate kinase
Phosphoglycerate kinase is a transferase enzyme used in the seventh step of glycolysis. It transfers a phosphate group from 1,3-bisphosphoglycerate to ADP, forming ATP and 3-Phosphoglycerate....
, pyruvate kinase
Pyruvate kinase
Pyruvate kinase is an enzyme involved in glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate to ADP, yielding one molecule of pyruvate and one molecule of ATP.-Reaction:The reaction with pyruvate kinase:...
and creatine kinase
Creatine kinase
Creatine kinase , also known as creatine phosphokinase or phospho-creatine kinase , is an enzyme expressed by various tissues and cell types. CK catalyses the conversion of creatine and consumes adenosine triphosphate to create phosphocreatine and adenosine diphosphate...
, resulting in formation of respective antivirally active triphosphate analogues.
In their respective triphosphate forms, NRTIs and the only NtRTI available compete with their corresponding endogenous deoxynucleotide triphosphate (dNTPs) for incorporation into the nascent DNA chain (see figure 1). Unlike dNTPs substrate, NRTIs lack a 3´-hydroxyl group on the deoxyribose
Deoxyribose
Deoxyribose, more, precisely 2-deoxyribose, is a monosaccharide with idealized formula H---3-H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of an oxygen atom...
moiety. Once incorporated into the DNA chain, the absence of a 3´-hydroxyl group, which normally forms the 5´- to 3´- phosphoester bond with the next nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
, blocks further extension of the DNA by RT, and they act as chain terminators.
First step towards treatment of HIV- zidovudine
In 1964 zidovudine (AZT) was synthesized by Horwitz at the Michigan Cancer Foundation. The 3´hydroxyl group in the deoxyribose ring of thymidine is replaced by an azido group which gives us zidovudine. The lack of the 3´hydroxyl group which provides the attachment point for the next nucleotide in the growing DNA chain during the reverse transcription makes it an obligate chain terminator. Ziduvodine is incorporated in place of thymidine and is an extremely potent inhibitor of HIV replicationSelf-replication
Self-replication is any behavior of a dynamical system that yields construction of an identical copy of that dynamical system. Biological cells, given suitable environments, reproduce by cell division. During cell division, DNA is replicated and can be transmitted to offspring during reproduction...
. This compound had been prepared in 1964 as a potential anti-cancer agent but was shown to be ineffective. In 1974 zidovudine was reported to have activity against retroviruses and was subsequently re-screened as an antiviral when the AIDS epidemic hit Western societies during mid 1980‘s. However, ziduvodine is relatively toxic since it is converted into the triphosphate by the cellular enzymes and therefore it is activated in uninfected cells.
Dideoxynucleosides
| Dideoxyadenosine | Didanosine | |
|---|---|---|
| Chemical structure |
Dideoxynucleosides are analogues of nucleoside where the sugar ring lacks both 2´ and 3´-hydroxyl groups. Three years after the synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of zidovudine, Jerome Horwitz and his colleagues in Chicago prepared another dideoxynucleoside now known as zalcitabine (ddC). Zalcitabine is a synthetic pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
nucleoside analogue, structurally related to deoxycytidine
Deoxycytidine
Deoxycytidine is a deoxyribonucleoside. It is like cytidine, but with one oxygen atom removed....
, in which the 3´-hydroxyl group of the ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
sugar moiety is substituted with hydrogen. Zalcitabine was approved by the FDA for the treatment of HIV-1 in June 1992.
2´,3´-dideoxyinosine or didanosine
Didanosine
Didanosine is sold under the trade names Videx and Videx EC. It is a reverse transcriptase inhibitor, effective against HIV and used in combination with other antiretroviral drug therapy as part of highly active antiretroviral therapy .-History:The related pro-drug of didanosine,...
is converted into dideoxyadenosine in vivo. Its development has a long history. In 1964 dideoxyadenosine, the corresponding adenosine analogue of zalcitabine was synthesised. Dideoxyadenosine caused kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
damage so didanosine was prepared from dideoxyadenosine by enzymatic oxidation (see table 1). It was found to be active against HIV without causing kidney damage. Didanosine was approved by the FDA for the treatment of HIV-1 in October 1991.
Zalcitabine and didanosine are both obligate chain terminators, that have been developed for anti-HIV treatment. Unfortunately, both drugs lack selectivity
Selectivity
Selectivity may refer to:* Selectivity , in radio transmission* Binding selectivity, in pharmacology* Functional selectivity, in pharmacology* Socioemotional selectivity theory, in social psychology...
and therefore cause side-effects.
Further modification of the dideoxy framework led to the development of 2´,3´-didehydro-3´-deoxythymidine (stavudine, d4T). Activity of stavudine was shown to be similar to that of zidovudine, although their phosphorylation patterns differ; the
affinity
Affinity
Affinity is a word used in a variety of fields, usually to indicate some kind of preference, relationship, or a potential or actual closeness between two entities.Articles dealing with various usages of the word: affinity include:-Commerce and law:...
for zidovudine to thymidine kinase
Thymidine kinase
Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, . It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2...
(the enzyme responsible for the first phosphorylation) is similar to that of thymidine
Thymidine
Thymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA...
, whereas the affinity for stavudine is 700-fold weaker.
| Zalcitabine | Lamivudine | |
|---|---|---|
| Chemical structure |
2',3'‐dideoxy‐3'‐thiacytidine (lamivudine, 3TC) was discovered by Bernard Belleau
Bernard Belleau
Bernard Belleau, OC, FRSC was a Canadian molecular pharmacologist best known for his role in the discovery of Lamivudine, a drug used in the treatment of HIV and Hepatitis B infection....
. The history of lamivudine can be traced back to the mid‐seventies while Bernard Belleau was investigating sugar derivative
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
s. Lamivudine was developed as the sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
analogue of zalcitabine (see table 2). It was initially synthesized as a racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
mixture (BCH-189) and analysis showed that both positive and negative enantiomers of BCH-189 had in vitro activity against HIV. Lamivudine which is the negative enantiomer of 2',3'‐dideoxy‐3'‐thiacytidine and is a pyrimidine nucleoside analogue. The 3' carbon of the ribose ring of 2'-deoxycytidine has been replaced by a sulfur atom because it had greater anti-HIV activity and is less toxic than the positive enantiomer.
Next in line was 2',3'‐dideoxy‐5-fluoro-3'‐thiacytidine (Emtricitabine, FTC) which is
a structural homologue
Homology (chemistry)
In chemistry, homology refers to the appearance of homologues. A homologue is a compound belonging to a series of compounds differing from each other by a repeating unit, such as a methylene group, a peptide residue, etcetera....
of lamivudine. The structural difference is the 5-fluoro-modification of the base moiety of lamivudine. It is similar in many ways to lamivudine and is active against both HIV-1 and hepatitis B virus (HBV
Hepatitis B virus
Hepatitis B is an infectious illness caused by hepatitis B virus which infects the liver of hominoidea, including humans, and causes an inflammation called hepatitis. Originally known as "serum hepatitis", the disease has caused epidemics in parts of Asia and Africa, and it is endemic in China...
).
Carbocyclic nucleoside
Carbocyclic analogues of dideoxyadenosine were investigated for their anti-HIV activity. Minimal activity was first observed. Many nucleoside analogues were prepared and examined but only one had significant activity and satisfied the requirements for clinicalClinical trial
Clinical trials are a set of procedures in medical research and drug development that are conducted to allow safety and efficacy data to be collected for health interventions...
use. That was 2´,3´-didehydro analogue of dideoxyadenosine. Insertion of a cyclopropyl group on its 6-amino nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
of the adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
ring increased lipophilicity and thus enhanced brain penetration. The resulting compound is known as abacavir (see table 3). Abacavir was approved by the FDA for use in therapy of HIV-1 infections in December 1998.
This drug is the only approved antiretroviral that is active as a guanosine
Guanosine
Guanosine is a purine nucleoside comprising guanine attached to a ribose ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate , cyclic guanosine monophosphate , guanosine diphosphate , and guanosine triphosphate...
analogue in vivo. First it is monophosphorylated by adenosine phosphotransferase and then the monophosphate is converted to carbovir 3´-monophosphate. Subsequently it is fully phosphorylated and the carbovir is incorporated by the RT into the DNA chain and acts as a chain terminator. Carbovir is a related guanosine analogue that had poor oral bioavailability
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is used to describe the fraction of an administered dose of unchanged drug that reaches the systemic circulation, one of the principal pharmacokinetic properties of drugs. By definition, when a medication is administered...
and thus was withdrawn from clinical development.
| Dideoxyadenosine | Didanosine | Abacavir | |
|---|---|---|---|
| Chemical structure |
Acyclic nucleotide – the only approved NtRTI
Nucleotide analogues require only two phosphorlylation steps whereas nucleoside analogues require three steps. ReductionReduction
Reduction, reduced, or reduce may refer to:- Chemistry :* Reduction, part of a reduction-oxidation reaction where oxygen is being removed from a compound.** Reduced gas, a gas with a low oxidation number...
in the phosphorylation requirement may allow more rapid and complete conversion of drugs to their active metabolites. Such considerations have led to the development of phosphonate nucleotide analogues such as tenofovir. Tenofovir disoproxil fumarate (Tenofovir DF) is the prodrug
Prodrug
A prodrug is a pharmacological substance administered in an inactive form. Once administered, the prodrug is metabolised in vivo into an active metabolite, a process termed bioactivation. The rationale behind the use of a prodrug is generally for absorption, distribution, metabolism, and...
of tenofovir. Tenofovir is an acyclic adenosine derivative. The acyclic nature of the compound and its phosphonate moiety are unique structural features among the approved NRTIs. Tenofovir DF is hydrolyzed enzymatically to tenofovir which exhibits anti-HIV activity. It was developed by the synthesis and broad spectrum antiviral activity of 2,3-dihydroxypropyladenine. Tenofovir DF was the first nucleotide reverse-transcriptase inhibitor approved by the FDA for the treatment of HIV-1 infection in October 2001.
| Nucleotide analogue | Nucleoside analogues | |||||||
|---|---|---|---|---|---|---|---|---|
Purine analogues |
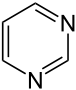 Pyrimidine analogues |
Purine analogues |
||||||
| Nucleoside | Adenosine |
Deoxythymidine |
Deoxycytidine |
Adenosine |
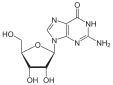 Guanosine |
|||
| Drug | 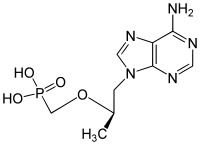 Tenofovir ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic |
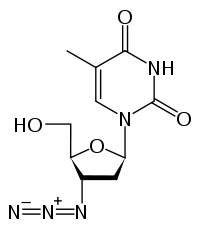 Zidovudine 3´Azido-2´,3´-dideoxythymidine, azidothy midine (AZT) |
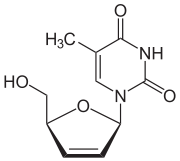 Stavudine 2´,3´-Didehydro-2´,3´-dideoxythymidine (d4T) |
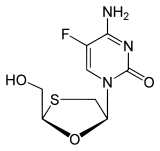 Emtricitabine (-)-ß-L-3´-thia-2´,3´-dideoxy-5-fluorocytidine ( (-)FTC) |
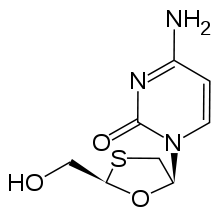 Lamivudine 2´,3´-Dideoxy-3´-thiacytidine (3TC) |
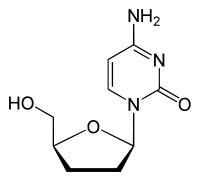 Zalcitabine 2´,3´-Dideoxycycytine (ddC) |
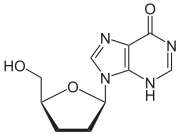 Didanosine 2´,3´-Dideoxyinosine (ddI) |
 Abacavir (4-(2-amino-6-(cyclopropylamino)-9H-purin-9yl) cyclopent-2enyl)methanol(ABC) |
Resistance
Currently, appearance of drug resistant viruses is an inevitable consequence of prolonged exposure of HIV-1 to antiretroviral therapy. Drug resistance is a serious clinical concern in treatment of viral infection, and it is a particularly difficult problem in treatment of HIV. Resistance mutations are known for all approved NRTIs.Two main mechanisms are known that cause NRTI drug resistance: Interference with the incorporation of NRTIs and excision of incorporated NRTIs.Interference with the incorporated NRTIs involves a mutation
Mutation
In molecular biology and genetics, mutations are changes in a genomic sequence: the DNA sequence of a cell's genome or the DNA or RNA sequence of a virus. They can be defined as sudden and spontaneous changes in the cell. Mutations are caused by radiation, viruses, transposons and mutagenic...
in the p66 subdomain of the RT.The mutation causes a steric hindrance that can exclude certain drugs, for example lamivudine, from being incorporated during reverse transcription. In case of excision of incorporated NRTIs the resistant enzymes readily accept the inhibitor as a substrate for incorporation into the DNA chain. Subsequently the RT enzyme can remove the incorporated NRTI by reversing the polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
step. The excision reaction requires a pyrophosphate donor which RT joins to the NRTI at the 3´primer terminus, excising it from the primer DNA.
To achieve efficient inhibition of HIV-1 replication in patients, and to delay or prevent appearance of drug resistant viruses, drug combinations are used. HAART, also known as highly active antiretroviral therapy consists of combinations of antiviral drugs which include NRTIs, NtRTI, non-nucleoside reverse-transcriptase inhibitors and protease inhibitors.
Current status
Currently, there are several NRTIs in various stages of clinical and preclinical development. The main reasons for continuing the search for new NRTIs against HIV-1 are to decrease toxicity, increase efficiency against resistant viruses, and simplify anti-HIV-1 treatment.Apricitabine (ATC)
Apricitabine is a deoxycytidine analogue. It is structurally related to lamivudine where the positions of the oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and the sulfur are essentially reversed. Even though apricitabine is a little less potent in vitro compared to some other NRTIs, it maintains its activity against a broad spectrum of HIV-1 variants with NRTI resistance mutations. Apricitabine is in the final stage of clinical development for the treatment of NRTI-experienced patients.
Elvucitabine (L-d4FC)
ElvucitabineElvucitabine
Elvucitabine is an experimental nucleoside reverse transcriptase inhibitor , developed by Achillion Pharmaceuticals, Inc. for the treatment of HIV.Currently, it is in Phase II clinical trials....
is a deoxycytidine analogue with activity against HIV resistant to several other nucleoside analogues, including zidovudine and lamivudine. This is partly because of high intracellular
Intracellular
Not to be confused with intercellular, meaning "between cells".In cell biology, molecular biology and related fields, the word intracellular means "inside the cell".It is used in contrast to extracellular...
levels of its triphosphate metabolite
Metabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
reached in cells. Clinical trials of elvucitabine are on hold, because it has shown bone marrow suppression
Bone marrow suppression
Bone marrow suppression or myelotoxicity or myelosuppression, is the decrease in cells responsible for providing immunity, carrying oxygen, and those responsible for normal blood clotting is a serious side effect of chemotherapy and certain drugs affecting the immune system such as azathioprine...
in some patients, with CD4+ cell numbers dropping as early as two days after initiation of dosing.
Amdoxovir (DAPD)
AmdoxovirAmdoxovir
Amdoxovir is an nucleoside reverse transcriptase inhibitor undergoing research for the treatment of HIV. It was discovered by R.F. Schinazi and C.K. Chu . It is being developed by RFS Pharma. Currently, it is in Phase II clinical studies.-External links:...
is a guanosine analogue NRTI prodrug that has good bioavailability.It is deaminated intracellularly by adenosine deaminase
Adenosine deaminase
Adenosine deaminase is an enzyme involved in purine metabolism. It is needed for the breakdown of adenosine from food and for the turnover of nucleic acids in tissues.-Reactions:...
to dioxolane
Dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula 2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane is an peroxide...
guanine (DXG). DXG-triphosphate, the active form of the drug, has greater activity than DAPD-triphosphate. Amdoxovir is currently in phasa II clinical trials.
Racivir (RCV)
RacivirRacivir
Racivir is an experimental nucleoside reverse transcriptase inhibitor , developed by Pharmasset for the treatment of HIV. It is the enantiomer of emtricitabine, meaning that the two compounds are mirror images of each other....
is a racemic mixture of the two β-enantiomers of emtricitabine (FTC), (-)-FTC and (+)-FTC. Racivir has excellent oral bioavailability and has the advantage of needing to be taken only once a day. Racivir can be considered to be used in combination of two NRTIs and has shown promising antiviral activity when used in combination. Racivir is currently in phase II clinical trials.
| Drug candidate | Apricitabine | Elvucitabine | Amdoxovir | Racivir |
|---|---|---|---|---|
| Chemical structure | 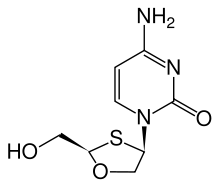 |
|||
| Phase of development | Final stage of clinical development | On hold | phase II | phase II |
There are several more NRTIs in development. Either the sponsors have filed for an Investigational New Drug
Investigational New Drug
The United States Food and Drug Administration's Investigational New Drug program is the means by which a pharmaceutical company obtains permission to ship an experimental drug across state lines before a marketing application for the drug has been approved...
(IND) application, the application has been approved by the FDA or the drugs are in different phases of clinical trails. Some of the NRTIs that are in development exhibit various attractive pharmacological properties that could make them desirable for the treatment of patients in need of new agents.
See also
- Antiretroviral drugAntiretroviral drugAntiretroviral drugs are medications for the treatment of infection by retroviruses, primarily HIV. When several such drugs, typically three or four, are taken in combination, the approach is known as Highly Active Antiretroviral Therapy, or HAART...
- Discovery and development of CCR5 receptor antagonistsDiscovery and development of CCR5 receptor antagonistsCCR5 receptor antagonists are a class of small molecules that antagonize the CCR5 receptor. The C-C motif chemokine receptor CCR5 is involved in the HIV entry process...
- Discovery and Development of Non-Nucleoside Reverse-Transcriptase Inhibitors
- Discovery and Development of HIV Protease InhibitorsDiscovery and development of HIV protease inhibitorsMany major physiological processes depend on regulation of proteolytic enzyme activity and there can be dramatic consequences when equilibrium between an enzyme and its substrates is disturbed. In this prospective, the discovery of small-molecule ligands, like protease inhibitors, that can modulate...
- Reverse-transcriptase inhibitor
- Protease inhibitorProtease inhibitor (pharmacology)Protease inhibitors are a class of drugs used to treat or prevent infection by viruses, including HIV and Hepatitis C. PIs prevent viral replication by inhibiting the activity of proteases, e.g.HIV-1 protease, enzymes used by the viruses to cleave nascent proteins for final assembly of new...
- Entry inhibitor
- Discovery and development of HIV protease inhibitorsDiscovery and development of HIV protease inhibitorsMany major physiological processes depend on regulation of proteolytic enzyme activity and there can be dramatic consequences when equilibrium between an enzyme and its substrates is disturbed. In this prospective, the discovery of small-molecule ligands, like protease inhibitors, that can modulate...
- Discovery and development of CCR5 receptor antagonistsDiscovery and development of CCR5 receptor antagonistsCCR5 receptor antagonists are a class of small molecules that antagonize the CCR5 receptor. The C-C motif chemokine receptor CCR5 is involved in the HIV entry process...
- Discovery and development of Bcr-Abl tyrosine kinase inhibitors

