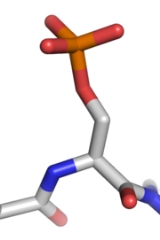
Phosphorylation
Encyclopedia
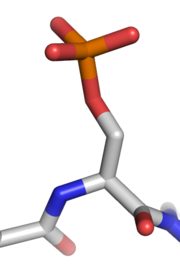
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
(PO43-) group to a protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
or other organic molecule. Phosphorylation activates or deactivates many protein enzymes.
Protein phosphorylation in particular plays a significant role in a wide range of cellular processes. Its prominent role in biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
is the subject of a very large body of research (as of March 2009, the Medline
MEDLINE
MEDLINE is a bibliographic database of life sciences and biomedical information. It includes bibliographic information for articles from academic journals covering medicine, nursing, pharmacy, dentistry, veterinary medicine, and health care...
database returns nearly 160,000 articles on the subject, largely on protein phosphorylation).
History
In 1906, Phoebus A. Levene at the Rockefeller Institute for Medical Research identified phosphate in the protein vitellin (phosvitin), and by 1933 had detected phosphoserine in caseinCasein
Casein is the name for a family of related phosphoprotein proteins . These proteins are commonly found in mammalian milk, making up 80% of the proteins in cow milk and between 60% and 65% of the proteins in human milk....
, with Fritz Lipmann. However, it took another 20 years before Eugene P. Kennedy described the first ‘enzymatic phosphorylation of proteins’.
Function
Reversible phosphorylation of proteins is an important regulatory mechanism that occurs in both prokaryoticProkaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
and eukaryotic
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
organisms.
Enzymes called kinases (phosphorylation) and phosphatases (dephosphorylation) are involved in this process. Many enzymes and receptors are switched "on" or "off" by phosphorylation and dephosphorylation. Reversible phosphorylation results in a conformational change
Protein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
in the structure in many enzymes and receptors, causing them to become activated or deactivated. Phosphorylation usually occurs on serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
, threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
and histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residues in eukaryotic proteins. Histidine phosphorylation of eukaryotic proteins appears to be much more frequent than tyrosine phosphorylation. In prokaryotic proteins phosphorylation occurs on the serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
, threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
or arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
or lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
residues. The addition of a phosphate (PO4) molecule to a polar R group of an amino acid residue can turn a hydrophobic portion of a protein into a polar and extremely hydrophilic portion of molecule. In this way it can introduce a conformational change in the structure of the protein via interaction with other hydrophobic and hydrophilic residues in the protein.
One such example of the regulatory role that phosphorylation plays is the p53 tumor suppressor protein. The p53 protein is heavily regulated and contains more than 18 different phosphorylation sites. Activation of p53 can lead to cell cycle arrest, which can be reversed under some circumstances, or apoptotic cell death. This activity occurs only in situations wherein the cell is damaged or physiology is disturbed in normal healthy individuals.
Upon the deactivating signal, the protein becomes dephosphorylated again and stops working. This is the mechanism in many forms of signal transduction
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
, for example the way in which incoming light is processed in the light-sensitive cells of the retina
Retina
The vertebrate retina is a light-sensitive tissue lining the inner surface of the eye. The optics of the eye create an image of the visual world on the retina, which serves much the same function as the film in a camera. Light striking the retina initiates a cascade of chemical and electrical...
.
Regulatory roles of phosphorylation include
- Biological thermodynamicsBiological thermodynamicsBiological thermodynamics is a phrase that is sometimes used to refer to bioenergetics, the study of energy transformation in the biological sciences...
of energy-requiring reactions- Phosphorylation of Na+/K+-ATPaseNa+/K+-ATPaseNa+/K+-ATPase is an enzyme located in the plasma membrane in all animals.- Sodium-potassium pumps :Active transport is responsible for cells containing relatively high...
during the transport of sodium (Na+) and potassium (K+) ions across the cell membrane in osmoregulationOsmoregulationOsmoregulation is the active regulation of the osmotic pressure of an organism's fluids to maintain the homeostasis of the organism's water content; that is it keeps the organism's fluids from becoming too diluted or too concentrated. Osmotic pressure is a measure of the tendency of water to move...
to maintain homeostasis of the body's water content.
- Phosphorylation of Na+/K+-ATPase
- Mediates enzyme inhibitionEnzyme inhibitorAn enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
- Phosphorylation of the enzyme GSK-3GSK-3Glycogen synthase kinase 3 is a serine/threonine protein kinase that mediates the addition of phosphate molecules on certain serine and threonine amino acids in particular cellular substrates...
by AKTAKTAkt, also known as Protein Kinase B , is a serine/threonine protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription and cell migration.-Family members:...
(Protein kinase B) as part of the insulin signaling pathway. - Phosphorylation of srcSrc (gene)Proto-oncogene tyrosine-protein kinase Src is an enzyme that in humans is encoded by the SRC gene.Src is a proto-oncogene encoding a tyrosine kinase originally discovered by J. Michael Bishop and Harold E. Varmus, for which they won the 1989 Nobel Prize in Physiology or Medicine. It belongs to a...
tyrosine kinase by C-terminal Src kinase (Csk) induces a conformational change in the enzyme, resulting in a fold in the structure, which masks its kinase domain, and is thus shut "off".
- Phosphorylation of the enzyme GSK-3
- Important for protein-protein interactionProtein-protein interactionProtein–protein interactions occur when two or more proteins bind together, often to carry out their biological function. Many of the most important molecular processes in the cell such as DNA replication are carried out by large molecular machines that are built from a large number of protein...
via "recognition domainsProtein domainA protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
."- Phosphorylation of the cytosolic components of NADPH oxidaseNADPH oxidaseThe NADPH oxidase is a membrane-bound enzyme complex. It can be found in the plasma membrane as well as in the membrane of phagosome.-Subunits:It is made up of six subunits...
, a large membrane-bound, multi-protein enzyme present in phagocytic cellsPhagocytePhagocytes are the white blood cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are...
, plays an important role in the regulation of protein-protein interactions in the enzyme.
- Phosphorylation of the cytosolic components of NADPH oxidase
- Important in protein degradation.
- In the late 1990s, it was recognized that phosphorylation of some proteins causes them to be degraded by the ATP-dependent ubiquitinUbiquitinUbiquitin is a small regulatory protein that has been found in almost all tissues of eukaryotic organisms. Among other functions, it directs protein recycling.Ubiquitin can be attached to proteins and label them for destruction...
/proteasomeProteasomeProteasomes are very large protein complexes inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, they are located in the nucleus and the cytoplasm. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks...
pathway. These target proteins become substrates for particular E3 ubiquitinUbiquitinUbiquitin is a small regulatory protein that has been found in almost all tissues of eukaryotic organisms. Among other functions, it directs protein recycling.Ubiquitin can be attached to proteins and label them for destruction...
ligases only when they are phosphorylated.
- In the late 1990s, it was recognized that phosphorylation of some proteins causes them to be degraded by the ATP-dependent ubiquitin
Signaling networks
Elucidating complex signaling pathway phosphorylation events can be difficult. In a cellular signaling pathways, a protein A phosphorylates protein B, and B phosphorylates C. However, in another signaling pathway, protein D phosphorylates A, or phosphorylates protein C. Global approaches such as phosphoproteomicsPhosphoproteomics
Phosphoproteomics is a branch of proteomics that identifies, catalogs, and characterizes proteins containing a phosphate group as a post-translational modification. Phosphorylation is a key reversible modification that regulates protein function, subcellular localization, complex formation,...
, the study of phosphorylated proteins, which is a sub-branch of proteomics
Proteomics
Proteomics is the large-scale study of proteins, particularly their structures and functions. Proteins are vital parts of living organisms, as they are the main components of the physiological metabolic pathways of cells. The term "proteomics" was first coined in 1997 to make an analogy with...
, combined with mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
-based proteomics
Proteomics
Proteomics is the large-scale study of proteins, particularly their structures and functions. Proteins are vital parts of living organisms, as they are the main components of the physiological metabolic pathways of cells. The term "proteomics" was first coined in 1997 to make an analogy with...
, have been utilised to identify and quantify dynamic changes in phosphorylated proteins over time. These techniques are becoming increasingly important for the systematic analysis of complex phosphorylation networks. They have been successfully used to identify dynamic changes in the phosphorylation status of more than 6000 sites after stimulation with epidermal growth factor
Epidermal growth factor
Epidermal growth factor or EGF is a growth factor that plays an important role in the regulation of cell growth, proliferation, and differentiation by binding to its receptor EGFR...
.
Another approach for understanding Phosphorylation Network, is by measuring the genetic interactions between multiple phosphorylating proteins and their targets. This reveals interesting recurring patterns of interactions - network motifs.
Protein phosphorylation sites
There are thousands of distinct phosphorylation sites in a given cell since:1) There are thousands of different kinds of proteins in any particular cell (such as a lymphocyte
Lymphocyte
A lymphocyte is a type of white blood cell in the vertebrate immune system.Under the microscope, lymphocytes can be divided into large lymphocytes and small lymphocytes. Large granular lymphocytes include natural killer cells...
).
2) It is estimated that 1/10 to 1/2 of proteins are phosphorylated (in some cellular state).
3) Phosphorylation often occurs on multiple distinct sites on a given protein.
Since phosphorylation of any site on a given protein can change the function or localization of that protein, understanding the "state" of a cell requires knowing the phosphorylation state of its proteins. For example, if amino acid Serine-473 ("S473") in the protein AKT
AKT
Akt, also known as Protein Kinase B , is a serine/threonine protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription and cell migration.-Family members:...
is phosphorylated, AKT
AKT
Akt, also known as Protein Kinase B , is a serine/threonine protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription and cell migration.-Family members:...
is, in general, functionally active as a kinase. If not, it is an inactive kinase.
Types of phosphorylation
See also kinaseKinase
In chemistry and biochemistry, a kinase is a type of enzyme that transfers phosphate groups from high-energy donor molecules, such as ATP, to specific substrates, a process referred to as phosphorylation. Kinases are part of the larger family of phosphotransferases...
s for more details on the different types of phosphorylation
Within a protein, phosphorylation can occur on several amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. Phosphorylation on serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
is the most common, followed by threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
. Tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
phosphorylation is relatively rare. However, since tyrosine phosphorylated proteins are relatively easy to purify using antibodies, tyrosine phosphorylation sites are relatively well understood. Histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
and aspartate phosphorylation occurs in prokaryotes as part of two-component signaling and in some cases in eukaryotes in some signal transduction pathways.
Detection and characterization
Antibodies can be used as powerful tools to detect whether a protein is phosphorylated at a particular site. Antibodies bind to and detect phosphorylation-induced conformational changes in the protein. Such antibodies are called phospho-specific antibodies; hundreds of such antibodies are now available. They are becoming critical reagents both for basic research and for clinical diagnosis.
Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis, abbreviated as 2-DE or 2-D electrophoresis, is a form of gel electrophoresis commonly used to analyze proteins...
. Indeed, phosphorylation replaces neutral hydroxyl groups on serines, threonines, or tyrosines with negatively-charged phosphates with pKs near 1.2 and 6.5. Thus, below pH 5.5, phosphates add a single negative charge; near pH 6.5, they add 1.5 negative charges; above pH 7.5, they add 2 negative charges. The relative amount of each isoform can also easily and rapidly be determined from staining intensity on 2D gels.
In some very specific cases, the detection of the phosphorylation as a shift in the protein's electrophoretic mobility is possible on simple 1-dimensional SDS-PAGE gels, as it's described for instance for a transcriptional coactivator by Kovacs et al. Strong phosphorylation-related conformational changes (that persist in detergent-containing solutions) are thought to underlie this phenomenon. Most of the phosphorylation sites for which such a mobility shift has been described fall in the category of SP and TP sites (i.e. a proline residue follows the phosphorylated serine or threonine residue).
More recently large-scale mass spectrometry analyses have been used to determine sites of protein phosphorylation. Over the last 4 years, dozens of studies have been published, each identifying thousands of sites, many of which were previously undescribed. Mass spectrometry is ideally suited for such analyses, as the addition of phosphorylation results in an increase in the mass of the protein and the phosphorylated residue. However, advanced, highly accurate mass spectrometers are needed for these studies, limiting the technology to labs with high-end mass spectrometers.
A detailed characterization of the sites of phosphorylation is very difficult, and the quantitation of protein phosphorylation by mass spectrometry requires isotopic internal standard approaches. A relative quantitation can be obtained with a variety of differential isotope labeling technologies. There are also several quantitative protein phosphorylation methods, including fluorescence immunoassays, Microscale Thermophoresis
Microscale Thermophoresis
Microscale Thermophoresis is a technology for the analysis of biomolecules. Microscale Thermophoresis is the directed movement of particles in a microscopic temperature gradient...
, FRET, TRF, fluorescence polarization, fluorescence-quenching, mobility shift, bead-based detection, and cell-based formats.
Other kinds
ATPAdenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, the "high-energy" exchange medium in the cell, is synthesized in the mitochondrion
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
by addition of a third phosphate group to ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
in a process referred to as oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
. ATP is also synthesized by substrate-level phosphorylation
Substrate-level phosphorylation
Substrate-level phosphorylation is a type of metabolism that results in the formation and creation of adenosine triphosphate or guanosine triphosphate by the direct transfer and donation of a phosphoryl group to adenosine diphosphate or guanosine diphosphate from a phosphorylated reactive...
during glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
.
ATP is synthesized at the expense of solar energy by photophosphorylation
Photophosphorylation
The production of ATP using the energy of sunlight is called photophosphorylation. Only two sources of energy are available to living organisms: sunlight and reduction-oxidation reactions...
in the chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s of plant cells.
Phosphorylation of sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s is often the first stage of their catabolism
Catabolism
Catabolism is the set of metabolic pathways that break down molecules into smaller units and release energy. In catabolism, large molecules such as polysaccharides, lipids, nucleic acids and proteins are broken down into smaller units such as monosaccharides, fatty acids, nucleotides, and amino...
. It allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter.
External links
- Mammalian Phosphorylation Resource, which integrates information on available phospho-specific antibodies
- Functional analyses for site-specific phosphorylation of a target protein in cells (A Protocol)

