
Compounds of zinc
Encyclopedia
Compounds of zinc
are chemical compound
s containing the element zinc
which is a member of the group 12
of the periodic table
. The oxidation state
of most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element
with zinc(II) having much chemical behaviour in common with copper(II). Many salts of zinc(II) are isomorphous
with salts of magnesium(II) due to the ionic radii of the cations being almost the same. Zinc forms many complexes; metallo-proteins containing zinc are widespread in biological systems.
. The stereochemistry is tetrahedral and the bonds may be described as being formed from sp3 hybrid orbitals on the zinc ion. Examples occur in the oxide, ZnO, (calamine
) and sulfide, ZnS, (zinc blende) in which the oxide and sulfide ions are also tetrahedrally bound to four zinc ions. Many complexes, such as ZnCl42-, are tetrahedral. Tetrahedrally coordinated zinc is found in metallo-enzyme
s such as carbonic anhydrase
. However 6-coordinate complexes can also be formed by using empty 4d orbitals to form sp3d2 hybrid orbitals. The ion [Zn(H2O)6]2+, which is present when a zinc salt is dissolved in water, has an octahedral structure.
Many zinc(II) salts are isomorphous
(have the same type of crystal structure
) with the corresponding salts of magnesium
(II) which results from the fact that Zn2+ and Mg2+ have almost identical ionic radii. This comes about because of the d-block contraction
. Whilst calcium
is somewhat larger than magnesium, there is a steady decrease in size as atomic number increases from calcium to zinc. By chance it is the ionic radius of zinc that is almost equal to that of magnesium. In most other respects the chemistry of zinc(II) most closely resembles the chemistry of copper(II), its neighbour in the periodic table, in which there is less electron. However, whereas Cu2+ is classed as a transition metal ion by virtue of its electronic configuration, [Ar]3d9, in which there is an incomplete d-shell, Zn2+ is best considered to be an ion of a post-transition main group element
. The IUPAC periodic table
places zinc in the d-block
.
Some compounds with zinc in the oxidation state +1 are known. The compounds have the formula RZn2R and they contain a Zn — Zn bond analogous to the metal-metal bond in mercury(I) ion, Hg22+. In this respect zinc is similar to magnesium where low valent
compounds containing a Mg — Mg bond have been characterised.
No compounds of zinc in oxidation states other than +1 or +2 are known. Calculations indicate that a zinc compound with the oxidation state of +4 is unlikely to exist. Although higher oxidation states are more stable with the heavier elements of a group, the compound
was only characterized at 4 K in a neon/argon matrix.
 Zinc compounds, like those of main group element
Zinc compounds, like those of main group element
s, are mostly colourless. Exceptions occur when the compound contains a coloured anion or ligand
. Zinc selenide
, ZnSe, however, is yellow, due to charge-transfer
transitions and zinc telluride
, ZnTe is brown for the same reason. Zinc oxide turns yellow when heated due to the loss of some oxygen atoms and formation of a defect
structure.
Compounds containing zinc and no other metal are all diamagnetic.
with a standard redox potential of -0.76 V. Pure zinc tarnishes rapidly in air, eventually forming a passive layer of basic zinc carbonate, Zn5(OH)6CO3. The reaction of zinc with water is prevented by the passive layer. When this layer is penetrated by acid
s such as hydrochloric acid
and sulfuric acid
the reaction proceeds with the evolution of hydrogen gas.
The hydrogen ion is reduced by accepting an electron from the reducing agent. The zinc metal is oxidised. Amalgamation with mercury, as in the Jones reductor
also destroys the passive layer. Zinc reacts with alkali
s as with acids. It reacts directly with oxidising non-metals such as chalcogen
s and halogen
s to form binary compounds.
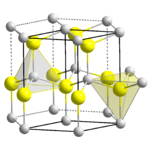
 Zinc oxide, ZnO
Zinc oxide, ZnO
, is the most important manufactured compound of zinc, with a wide variety of uses. It crystallizes with the Wurtzite structure. It is amphoteric, dissolving in acids to give the aqueous Zn2+ ion and in alkali to give the tetrahedral hydroxo complex, [Zn(OH)4]2-. Zinc hydroxide, Zn(OH)2 is also amphoteric.
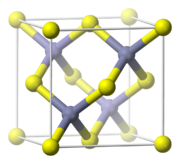
Zinc sulfide, ZnS
, crystallizes in two closely related structures, the Zinc blende structure
and the Wurtzite structure
which are common structures of compounds with the formula MA. Both Zn and S are tetrahedrally coordinated by the other ion. A useful property of ZnS is its phosphorescence
.The other chalcogen
ides, ZnSe
, and ZnTe
, have applications in electronics and optics.
Of the four halide
s
has the most ionic character, whereas the others,
,
, and
, have relatively low melting points and are considered to have more covalent character. The pnictogenides
(notable for its high melting point),
,
and
, have various applications. Other binary compounds of zinc include the peroxide
, the hydride
, and the carbide .
(used as oxidizing agent
, the chlorate
, the sulfate
(known as "white vitriol"), the phosphate
(used as primer
pigment
), the molybdate
(used as white pigment), , the chromate
(one of the few colored zinc compounds), the arsenite Zn(AsO2)2 (colorless powder) and the arsenate octahydrate (white powder, also referred to as koettigite are a few examples of other common inorganic compounds of zinc. The latter two compounds are both used in insecticides and wood preservatives. One of the simplest examples of an organic compound
of zinc is the acetate
, which has several medicinal applications. Zinc salts are usually fully dissociated
in aqueous solution. Exceptions occur when the anion can form a complex, such as in the case of zinc sulfate
, where the complex [Zn(H2O)n(SO4] may be formed, (log K
= ca. 2.5).
 ]
]
The most common structure of zinc complexes is tetrahedral which is clearly connected with the fact that the octet rule
is obeyed in these cases. Nevertheless, octahedral complexes comparable to those of the transition elements are not rare. Zn2+ is a class A acceptor in the classification of Ahrland, Chatt and Davies, and so forms stronger complexes with the first-row donor atoms oxygen or nitrogen than with second-row sulfur or phosphorus. In terms of HSAB theory Zn2+ is a hard acid.
In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species. Aqueous solutions of zinc salts are mildly acidic because the aqua-ion
is subject to hydrolysis with a pKa
of around 5, depending on conditions.
Hydrolysis explains why basic salts such as basic zinc acetate
and basic zinc carbonate, Zn3(OH)4(CO3).H2O are easy to obtain. The reason for the hydrolysis is the high electrical charge density on the zinc ion, which pulls electrons away from an OH bond of a coordinated water molecule and releases a hydrogen ion. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase
.
2.png) No fluoro complexes are known, but complexes with the other halides and with pseodohalides
No fluoro complexes are known, but complexes with the other halides and with pseodohalides
, [ZnX3]- and [ZnX4]2- can be prepared. The case of the thiocyanate
complex illustrates the class A character of the zinc ion as it is the N-bonded isomer, [Zn(NCS)4]2-in contrast to [Cd(SCN)4]2- which is S-bonded. Being a class-A acceptor does not preclude the formation of complexes with sulfur donors, as is shown by zinc dithiophosphate and the zinc finger complex (below).
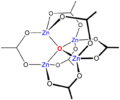
The acetylacetonate complex, Zn(acac)2 is interesting. As the ligand is bidentate a tetrahedral structure might be expected. However, the compound is in fact a trimer, Zn3(acac)6 in which each Zn ion is coordinated by five oxygen atoms in a distorted trigonal bipyramidal structure. Other 5-coordinate structures can be engineered by choosing ligands which have specific stereochemical requirements. For example, terpyridine
, which is a tridentate ligand forms the complex [Zn(terpy)Cl2]. Another example would involve a tripodal ligand such as Tris(2-aminoethyl)amine. The compound zinc cyanide, Zn(CN)2
, is not 2-coordinate. It adopts a polymeric structure consisting of tetrahedral zinc centres linked by bridging cyanide ligands. The cyanide group shows head to tail disorder with any zinc atom having between 1 and 4 carbon atom neighbours and the remaining being nitrogen atoms. These two examples illustrate the difficulty of sometimes relating structure to stoichiometry.
A coordination number of 2 occurs in the amide
Zn(NR1R2)2 (R1=CMe3, R2=SiMe3); the ligand is so bulky that there is not enough space for more than two of them.
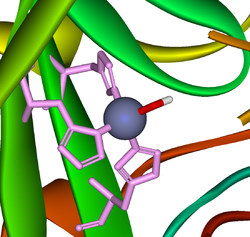
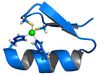 A very large number of metallo-enzyme
A very large number of metallo-enzyme
s contain zinc(II). Also many protein
s contain zinc for structural reasons. The zinc ion is invariably 4-coordinate with at least three ligands that are amino-acid side-chains. The imidazole
nitrogen of a histidine
side-chain is a common ligand. The following are typical examples of the two kinds of zinc-protein complexes.
In the active site of resting Carbonic anhydrase
a zinc ion is coordinated by three histidine residues. The fourth position is occupied by a water molecule, which is strongly polarized as in hydrolysis (see above). When carbon dioxide
enters the active site, it subject to nucleophilic attack by the oxygen atom which carries a partial negative charge, or indeed a full negative charge if the water molecule is dissociated. The CO2 is rapidly converted into a bicarbonate ion.
Some peptidases , such as Glutamate carboxypeptidase II
are thought to act in a similar way, with the zinc ion promoting the formation of a nucleophilic reagent.
The zinc finger
motif is a rigid substructure in a protein which facilitates the binding of the protein to another molecule such as DNA
. In this case all four coordination positions are occupied by the hystidine and cysteine
residues. The tetrahedral geometry around the zinc ion constrains an α helix
fragment and an antiparallel β sheet
fragment to a particular orientation with respect to each other.
The magnesium ion, which has a higher concentration in biological fluids, cannot perform these functions as its complexes are much weaker than those of zinc.
s contain zinc—carbon covalent bonds. Diethylzinc
was first reported in 1848. It was made by reaction of zinc and ethyl iodide
and is the first compound known to contain a metal—carbon sigma bond
. For a long time it was a mystery why copper(II) did not form an analogous compound. It was not until the 1980s that the reason was found: the zinc compound does not undergo the Beta-hydride elimination
reaction whereas the compound of the transition metal copper does so. Alkyl and aryl zinc compounds are contain the linear C—Zn—C motif. Because the zinc centre is coordinatively unsaturated the compound are powerful electrophile
s. In fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by reaction with water molecules. The use of zinc alkyls has been largely superseded by the use of the more easily handled Grignard reagents. This demonstrates yet another connection between the chemistries of zinc and magnesium.
Zinc cyanide,
, is used as a catalyst in some organic reactions.
Organometallic compounds of zinc(I) contain M—M bonds. decamethyldizincocene
is now known.
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
are chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s containing the element zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
which is a member of the group 12
Group 12 element
A group 12 element is one of the elements in group 12 in the periodic table. This includes zinc , cadmium and mercury . The further inclusion of copernicium in group 12 is supported by recent experiments on individual Cn atoms...
of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
. The oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element
Main group element
In chemistry and atomic physics, main group elements are elements in groups whose lightest members are represented by helium, lithium,...
with zinc(II) having much chemical behaviour in common with copper(II). Many salts of zinc(II) are isomorphous
Isomorphism (crystallography)
In crystallography crystals are described as isomorphous if they are closely similar in shape. Historically crystal shape was defined by measuring the angles between crystal faces with a goniometer...
with salts of magnesium(II) due to the ionic radii of the cations being almost the same. Zinc forms many complexes; metallo-proteins containing zinc are widespread in biological systems.
General characteristics
Zinc atoms have an electronic configuration of [Ar]3d104s2. When compounds in the +2 oxidation state are formed the s electrons are lost, so the bare zinc ion has the electronic configuration [Ar]3d10. This allows for the formation of four covalent bonds by accepting four electron pairs and thus obeying the octet ruleOctet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
. The stereochemistry is tetrahedral and the bonds may be described as being formed from sp3 hybrid orbitals on the zinc ion. Examples occur in the oxide, ZnO, (calamine
Calamine
Calamine is a mixture of zinc oxide with about 0.5% ferric oxide . It is the main ingredient in calamine lotion and is used as an antipruritic to treat mild pruritic conditions such as sunburn, eczema, rashes, poison ivy, chickenpox, and insect bites and stings...
) and sulfide, ZnS, (zinc blende) in which the oxide and sulfide ions are also tetrahedrally bound to four zinc ions. Many complexes, such as ZnCl42-, are tetrahedral. Tetrahedrally coordinated zinc is found in metallo-enzyme
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to...
s such as carbonic anhydrase
Carbonic anhydrase
The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst...
. However 6-coordinate complexes can also be formed by using empty 4d orbitals to form sp3d2 hybrid orbitals. The ion [Zn(H2O)6]2+, which is present when a zinc salt is dissolved in water, has an octahedral structure.
Many zinc(II) salts are isomorphous
Isomorphism (crystallography)
In crystallography crystals are described as isomorphous if they are closely similar in shape. Historically crystal shape was defined by measuring the angles between crystal faces with a goniometer...
(have the same type of crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
) with the corresponding salts of magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
(II) which results from the fact that Zn2+ and Mg2+ have almost identical ionic radii. This comes about because of the d-block contraction
D-block contraction
d-block contraction is a term used in chemistry to describe the effect of having full d orbitals on the period 4 elements. The elements in question are the Ga, Ge, As, Se and Br. Their electronic configurations include completely filled d orbitals...
. Whilst calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
is somewhat larger than magnesium, there is a steady decrease in size as atomic number increases from calcium to zinc. By chance it is the ionic radius of zinc that is almost equal to that of magnesium. In most other respects the chemistry of zinc(II) most closely resembles the chemistry of copper(II), its neighbour in the periodic table, in which there is less electron. However, whereas Cu2+ is classed as a transition metal ion by virtue of its electronic configuration, [Ar]3d9, in which there is an incomplete d-shell, Zn2+ is best considered to be an ion of a post-transition main group element
Main group element
In chemistry and atomic physics, main group elements are elements in groups whose lightest members are represented by helium, lithium,...
. The IUPAC periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
places zinc in the d-block
D-block
The d-block is the portion of the periodic table that contains the element groups 3-12. These groups correspond to the filling of the atomic d-orbital subshell, with electron configurations ranging from s2d1 to s2d10...
.
Some compounds with zinc in the oxidation state +1 are known. The compounds have the formula RZn2R and they contain a Zn — Zn bond analogous to the metal-metal bond in mercury(I) ion, Hg22+. In this respect zinc is similar to magnesium where low valent
Low valent magnesium compounds
Two low valent magnesium compounds have been discovered that are the first examples of stable magnesium compounds. Both examples have the formula L2Mg2, where L represents a bulky anionic ligand. X-ray crystallographic studies show an Mg-Mg bond length of 285.1 pm and 284.6 pm. Theoretical studies...
compounds containing a Mg — Mg bond have been characterised.
No compounds of zinc in oxidation states other than +1 or +2 are known. Calculations indicate that a zinc compound with the oxidation state of +4 is unlikely to exist. Although higher oxidation states are more stable with the heavier elements of a group, the compound
Mercury(IV) fluoride
Mercury fluoride, HgF4, is the first mercury compound to be discovered with the metal in the oxidation state IV. Mercury, like the other group 12 elements , has an s2d10 electron configuration and generally only forms bonds involving its s orbital...
was only characterized at 4 K in a neon/argon matrix.
Colour and magnetism

Main group element
In chemistry and atomic physics, main group elements are elements in groups whose lightest members are represented by helium, lithium,...
s, are mostly colourless. Exceptions occur when the compound contains a coloured anion or ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. Zinc selenide
Zinc selenide
Zinc selenide , is a light yellow binary solid compound. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C. ZnSe rarely occurs in nature...
, ZnSe, however, is yellow, due to charge-transfer
Charge-transfer
Charge-transfer may refer to:* Intervalence charge transfer* Charge-transfer complex* Charge-exchange ionization, a form of gas phase ionization...
transitions and zinc telluride
Zinc telluride
Zinc telluride is a binary chemical compound with the formula ZnTe. This solid is a semiconductor material with band gap of 2.23–2.25 eV. It is usually a P-type semiconductor. Its crystal structure is cubic, like that for sphalerite and diamond....
, ZnTe is brown for the same reason. Zinc oxide turns yellow when heated due to the loss of some oxygen atoms and formation of a defect
Frenkel defect
The Frenkel Defect is shown by ionic solids. The smaller ion is displaced from its lattice position to an interstitial site. It creates a vacancy defect at its original site and an interstitial defect at its new location.-Definition:...
structure.
Compounds containing zinc and no other metal are all diamagnetic.
Reactivity of the metal
Zinc is a strong reducing agentReducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
with a standard redox potential of -0.76 V. Pure zinc tarnishes rapidly in air, eventually forming a passive layer of basic zinc carbonate, Zn5(OH)6CO3. The reaction of zinc with water is prevented by the passive layer. When this layer is penetrated by acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s such as hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
the reaction proceeds with the evolution of hydrogen gas.
- Zn(s) + 2H+ (aq) → Zn2+ (aq) + H2 ↑
The hydrogen ion is reduced by accepting an electron from the reducing agent. The zinc metal is oxidised. Amalgamation with mercury, as in the Jones reductor
Jones reductor
A Jones reductor is a device which can be used to reduce a metal ion in aqueous solution to a very low oxidation state. The active component is a zinc/mercury amalgam...
also destroys the passive layer. Zinc reacts with alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
s as with acids. It reacts directly with oxidising non-metals such as chalcogen
Chalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
s and halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s to form binary compounds.
Binary compounds


Zinc oxide
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
, is the most important manufactured compound of zinc, with a wide variety of uses. It crystallizes with the Wurtzite structure. It is amphoteric, dissolving in acids to give the aqueous Zn2+ ion and in alkali to give the tetrahedral hydroxo complex, [Zn(OH)4]2-. Zinc hydroxide, Zn(OH)2 is also amphoteric.
Zinc sulfide, ZnS
Zinc sulfide
Zinc sulfide is a inorganic compound with the formula ZnS. ZnS is the main form of zinc in nature, where it mainly occurs as the mineral sphalerite...
, crystallizes in two closely related structures, the Zinc blende structure
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
and the Wurtzite structure
Wurtzite crystal structure
thumb|150px|right|General hexagonal crystal structureThe wurtzite crystal structure, named after the mineral wurtzite, is a crystal structure for various binary compounds. It is an example of a hexagonal crystal system....
which are common structures of compounds with the formula MA. Both Zn and S are tetrahedrally coordinated by the other ion. A useful property of ZnS is its phosphorescence
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
.The other chalcogen
Chalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
ides, ZnSe
Zinc selenide
Zinc selenide , is a light yellow binary solid compound. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C. ZnSe rarely occurs in nature...
, and ZnTe
Zinc telluride
Zinc telluride is a binary chemical compound with the formula ZnTe. This solid is a semiconductor material with band gap of 2.23–2.25 eV. It is usually a P-type semiconductor. Its crystal structure is cubic, like that for sphalerite and diamond....
, have applications in electronics and optics.
Of the four halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s
Zinc fluoride
Zinc fluoride is an inorganic chemical compound. It is encountered as the anydrous form and also as the tetrahydrate, ZnF2.4H2O . It has a high melting point and has the rutile structure containing 6 coordinate zinc, which suggests appreciable ionic character in its chemical bonding...
has the most ionic character, whereas the others,
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
,
Zinc bromide
Zinc bromide is a inorganic compound with the chemical formula ZnBr2. It is a colourless salt that shares many properties with zinc chloride , namely a high solubility in water forming acidic solutions, and solubility in organic solvents...
, and
Zinc iodide
Zinc iodide is a chemical compound of zinc and iodine, ZnI2. The anhydrous form is white and readily absorbs water from the atmosphere. It can be prepared by the direct reaction of zinc and iodine in refluxing ether...
, have relatively low melting points and are considered to have more covalent character. The pnictogenides
Zinc nitride
Zinc nitride is an inorganic compound of zinc and nitrogen. In pure form, it is cubic in structure.-Chemical properties:Zinc nitride can be obtained by thermally decomposing zincamide...
(notable for its high melting point),
Zinc phosphide
Zinc phosphide is an inorganic chemical compound.- Reactions :Zinc phosphide can be prepared by the reaction of zinc with phosphorus:Zinc phosphide will react with water to produce phosphine and zinc hydroxide :-Rodenticide:...
,
Zinc arsenide
Zinc arsenide is a binary compound of zinc with arsenic which forms gray tetragonal crystals....
and
Zinc antimonide
Zinc antimonide is an inorganic chemical compound. Like indium antimonide, aluminium antimonide, and gallium antimonide, it is a semiconducting intermetallic compound. It is used in transistors, infrared detectors and thermal imagers, as well as magnetoresistive devices....
, have various applications. Other binary compounds of zinc include the peroxide
Zinc peroxide
Zinc peroxide is a chemical compound used as a bleaching and curing agent. It appears as white to yellow powder. Perhaps its most important use is to promote cross-linking in carboxylated nitrile rubber and other elastomers. Another application of ZnO2 is additive to antiseptic ointments...
, the hydride
Zinc hydride
Zinc hydride is a chemical compound of zinc and hydrogen, ZnH2, which is used as a reducing agent in organic synthesis. First reported in 1947, it is a white crystalline powder when freshly made which turns grey if left at room temperature for a few days, presumably due to the decompostion to...
, and the carbide .
Salts
The nitrateZinc nitrate
Zinc nitrate is a highly deliquescent substance which is usually prepared by dissolving zinc in nitric acid. It can be used as a mordant in dyeing...
(used as oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
, the chlorate
Zinc chlorate
Zinc chlorate is an inorganic chemical compound used as a oxidizing agent in explosives....
, the sulfate
Zinc sulfate
Zinc sulfate is the inorganic compound with the formula ZnSO4 as well as any of three hydrates. It was historically known as "white vitriol". It is a colorless solid that is a common source of soluble zinc ions.-Production and reactivity:...
(known as "white vitriol"), the phosphate
Zinc phosphate
Zinc phosphate is an inorganic chemical compound used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment . Zinc phosphate coats better on a crystalline structure than bare metal, so a seeding agent is often used as a...
(used as primer
Primer (paint)
A primer is a preparatory coating put on materials before painting. Priming ensures better adhesion of paint to the surface, increases paint durability, and provides additional protection for the material being painted.-When primers are used:...
pigment
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
), the molybdate
Zinc molybdate
Zinc molybdate is an inorganic chemical compound. It is a white pigment, which can be used as corrosion inhibitor. While highly soluble molybdates like e.g. sodium molybdate are toxic in higher doses, zinc molybdate is essentially non-toxic because of its insolubility in water...
(used as white pigment), , the chromate
Zinc chromate
Zinc chromate, ZnCrO4, is a chemical compound containing the chromate anion, appearing as odorless yellow solid powder. It is used industrially in chromate conversion coatings, having been developed by Ford Motor Company in 1920s...
(one of the few colored zinc compounds), the arsenite Zn(AsO2)2 (colorless powder) and the arsenate octahydrate (white powder, also referred to as koettigite are a few examples of other common inorganic compounds of zinc. The latter two compounds are both used in insecticides and wood preservatives. One of the simplest examples of an organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
of zinc is the acetate
Zinc acetate
Zinc acetate is the chemical compound with the formula Zn2, which commonly occurs as a dihydrate Zn22. Both the hydrate and the anhydrous forms are colorless solids that are commonly used in chemical synthesis and as dietary supplements. Zinc acetates are prepared by the action of acetic acid on...
, which has several medicinal applications. Zinc salts are usually fully dissociated
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
in aqueous solution. Exceptions occur when the anion can form a complex, such as in the case of zinc sulfate
Zinc sulfate
Zinc sulfate is the inorganic compound with the formula ZnSO4 as well as any of three hydrates. It was historically known as "white vitriol". It is a colorless solid that is a common source of soluble zinc ions.-Production and reactivity:...
, where the complex [Zn(H2O)n(SO4] may be formed, (log K
Stability constants of complexes
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex...
= ca. 2.5).
Complexes

The most common structure of zinc complexes is tetrahedral which is clearly connected with the fact that the octet rule
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
is obeyed in these cases. Nevertheless, octahedral complexes comparable to those of the transition elements are not rare. Zn2+ is a class A acceptor in the classification of Ahrland, Chatt and Davies, and so forms stronger complexes with the first-row donor atoms oxygen or nitrogen than with second-row sulfur or phosphorus. In terms of HSAB theory Zn2+ is a hard acid.
In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species. Aqueous solutions of zinc salts are mildly acidic because the aqua-ion
Metal ions in aqueous solution
A metal ion in aqueous solution is a cation, dissolved in water, of chemical formula [Mn]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in rows 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have solvation...
is subject to hydrolysis with a pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of around 5, depending on conditions.
- [Zn(H2O)6]2+ [Zn(H2O)5(OH)]+ + H+
Hydrolysis explains why basic salts such as basic zinc acetate
Zinc acetate
Zinc acetate is the chemical compound with the formula Zn2, which commonly occurs as a dihydrate Zn22. Both the hydrate and the anhydrous forms are colorless solids that are commonly used in chemical synthesis and as dietary supplements. Zinc acetates are prepared by the action of acetic acid on...
and basic zinc carbonate, Zn3(OH)4(CO3).H2O are easy to obtain. The reason for the hydrolysis is the high electrical charge density on the zinc ion, which pulls electrons away from an OH bond of a coordinated water molecule and releases a hydrogen ion. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase
Carbonic anhydrase
The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst...
.
2.png)
Pseudohalogen
Pseudo'halogen molecules are inorganic molecules of the general formsPs–Ps or Ps–X, where Ps is a pseudohalogen group such as cyanide, cyanate, thiocyanate and others, and X is a "true" halogen...
, [ZnX3]- and [ZnX4]2- can be prepared. The case of the thiocyanate
Thiocyanate
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
complex illustrates the class A character of the zinc ion as it is the N-bonded isomer, [Zn(NCS)4]2-in contrast to [Cd(SCN)4]2- which is S-bonded. Being a class-A acceptor does not preclude the formation of complexes with sulfur donors, as is shown by zinc dithiophosphate and the zinc finger complex (below).
The acetylacetonate complex, Zn(acac)2 is interesting. As the ligand is bidentate a tetrahedral structure might be expected. However, the compound is in fact a trimer, Zn3(acac)6 in which each Zn ion is coordinated by five oxygen atoms in a distorted trigonal bipyramidal structure. Other 5-coordinate structures can be engineered by choosing ligands which have specific stereochemical requirements. For example, terpyridine
Terpyridine
Terpyridine is a heterocyclic compound derived from pyridine. This colourless solid is used as a ligand in coordination chemistry.-Synthesis:...
, which is a tridentate ligand forms the complex [Zn(terpy)Cl2]. Another example would involve a tripodal ligand such as Tris(2-aminoethyl)amine. The compound zinc cyanide, Zn(CN)2
Zinc cyanide
Zinc cyanide is the inorganic compound with the formula Zn2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.-Structure, properties, synthesis:...
, is not 2-coordinate. It adopts a polymeric structure consisting of tetrahedral zinc centres linked by bridging cyanide ligands. The cyanide group shows head to tail disorder with any zinc atom having between 1 and 4 carbon atom neighbours and the remaining being nitrogen atoms. These two examples illustrate the difficulty of sometimes relating structure to stoichiometry.
A coordination number of 2 occurs in the amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
Zn(NR1R2)2 (R1=CMe3, R2=SiMe3); the ligand is so bulky that there is not enough space for more than two of them.
Bio-complexes


Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to...
s contain zinc(II). Also many protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s contain zinc for structural reasons. The zinc ion is invariably 4-coordinate with at least three ligands that are amino-acid side-chains. The imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
nitrogen of a histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
side-chain is a common ligand. The following are typical examples of the two kinds of zinc-protein complexes.
In the active site of resting Carbonic anhydrase
Carbonic anhydrase
The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst...
a zinc ion is coordinated by three histidine residues. The fourth position is occupied by a water molecule, which is strongly polarized as in hydrolysis (see above). When carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
enters the active site, it subject to nucleophilic attack by the oxygen atom which carries a partial negative charge, or indeed a full negative charge if the water molecule is dissociated. The CO2 is rapidly converted into a bicarbonate ion.
- [(-hys)3Zn(H2O)]2+ + CO2 → [(-hys)3Zn]2+ + HCO3- + H+
Some peptidases , such as Glutamate carboxypeptidase II
Glutamate carboxypeptidase II
Glutamate carboxypeptidase II , also known as N-acetyl-L-aspartyl-L-glutamate peptidase I , NAAG peptidase, or Prostate specific membrane antigen is an enzyme that in humans is encoded by the FOLH1 gene...
are thought to act in a similar way, with the zinc ion promoting the formation of a nucleophilic reagent.
The zinc finger
Zinc finger
Zinc fingers are small protein structural motifs that can coordinate one or more zinc ions to help stabilize their folds. They can be classified into several different structural families and typically function as interaction modules that bind DNA, RNA, proteins, or small molecules...
motif is a rigid substructure in a protein which facilitates the binding of the protein to another molecule such as DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. In this case all four coordination positions are occupied by the hystidine and cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues. The tetrahedral geometry around the zinc ion constrains an α helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
fragment and an antiparallel β sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
fragment to a particular orientation with respect to each other.
The magnesium ion, which has a higher concentration in biological fluids, cannot perform these functions as its complexes are much weaker than those of zinc.
Organometallic compounds
Organozinc compoundOrganozinc compound
Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions....
s contain zinc—carbon covalent bonds. Diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
was first reported in 1848. It was made by reaction of zinc and ethyl iodide
Ethyl iodide
Ethyl iodide is a colorless, flammable chemical compound. It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus. On contact with air, especially on the effect of light, it decomposes and turns yellow or reddish from dissolved iodine.Ethyl iodide is very...
and is the first compound known to contain a metal—carbon sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
. For a long time it was a mystery why copper(II) did not form an analogous compound. It was not until the 1980s that the reason was found: the zinc compound does not undergo the Beta-hydride elimination
Beta-hydride elimination
Beta-hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the beta carbon. For instance butyl groups can undergo this reaction but methyl groups cannot...
reaction whereas the compound of the transition metal copper does so. Alkyl and aryl zinc compounds are contain the linear C—Zn—C motif. Because the zinc centre is coordinatively unsaturated the compound are powerful electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s. In fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by reaction with water molecules. The use of zinc alkyls has been largely superseded by the use of the more easily handled Grignard reagents. This demonstrates yet another connection between the chemistries of zinc and magnesium.
Zinc cyanide,
Zinc cyanide
Zinc cyanide is the inorganic compound with the formula Zn2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.-Structure, properties, synthesis:...
, is used as a catalyst in some organic reactions.
Organometallic compounds of zinc(I) contain M—M bonds. decamethyldizincocene
Decamethyldizincocene
Decamethyldizincocene is an organozinc compound with the formula [Zn22]. It is an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water...
is now known.
See also
- cadmium zinc tellurideCadmium zinc tellurideCadmium zinc telluride, or CZT, is a compound of cadmium, zinc and tellurium or, more strictly speaking, an alloy of cadmium telluride and zinc telluride. A direct bandgap semiconductor, it is used in a variety of applications, including radiation detectors, photorefractive gratings,...
- mercury cadmium tellurideMercury(II) cadmium(II) tellurideHgCdTe or mercury cadmium telluride is an alloy of CdTe and HgTe and is sometimes claimed to be the third semiconductor of technological importance after silicon and gallium arsenide...
- zinc gluconateZinc gluconateZinc gluconate is the zinc salt of gluconic acid. It is an ionic compound consisting of two moles of gluconate for each mole of zinc...
- zinc pyrithioneZinc pyrithioneZinc pyrithione is a coordination complex of zinc. This colourless solid is used as an antifungal and antibacterial agent. This coordination complex, which has many names, was first reported in the 1930s.- Structure of the compound :...
- zinc ricinoleateZinc ricinoleateZinc ricinoleate is the zinc salt of ricinoleic acid, a major fatty acid found in castor oil. It is used in many deodorants as an odor-adsorbing agent. The mechanism of this activity is unclear....
- zinc stearateZinc stearateZinc stearate is a zinc soap that repels water. It is insoluble in polar solvents such as alcohol and ether but soluble in aromatic hydrocarbons when heated. It is the most powerful mold release agent among all metal soaps. It contains no electrolyte and has a hydrophobic effect...
- Zinc pestZinc pestZinc pest, , is a destructive, intercrystalline corrosion process of zinc alloys containing lead impurities. It was first discovered to be a problem in 1923....

