
Amine oxide
Encyclopedia
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound
that contains the functional group
R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.
In the strict sense the term amine oxide applies only to oxides of tertiary amine
s. Sometimes it is also used for the analogous derivatives of primary and secondary amines.
Examples of amine oxides include pyridine N-oxide, a water-soluble crystalline solid with melting point
62-67°C, and N-methylmorpholine N-oxide
, which is an oxidant.
for amines and as chemical
intermediates. Long-chain alkyl amine oxides are used as nonionic surfactant
s and foam
stabilizers.
Amine oxides are highly polar molecules have a polarity
close to that of quaternary ammonium salts. Small amine oxides are very hydrophilic and have an excellent water solubility
and a very poor solubility in most organic solvent
s.
Amine oxides are weak bases
with a pKa
of around 4.5 that form R3N+-OH, cationic hydroxylamine
s, upon protonation
at a pH
below their pKa.
(H2O2), Caro's acid or peracids like mCPBA
in N-oxidation .
s of medication
and psychoactive drug
s. Examples include nicotine
, Zolmitriptan
, and morphine
.
Amine oxides of anti-cancer drugs
have been developed as prodrug
s that are metabolized in the oxygen
deficient cancer
tissue
to the active drug.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
that contains the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.
In the strict sense the term amine oxide applies only to oxides of tertiary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. Sometimes it is also used for the analogous derivatives of primary and secondary amines.
Examples of amine oxides include pyridine N-oxide, a water-soluble crystalline solid with melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
62-67°C, and N-methylmorpholine N-oxide
N-Methylmorpholine N-oxide
N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
, which is an oxidant.
Properties
Amine oxides are used as protecting groupProtecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
for amines and as chemical
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
intermediates. Long-chain alkyl amine oxides are used as nonionic surfactant
Surfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
s and foam
Foam
-Definition:A foam is a substance that is formed by trapping gas in a liquid or solid in a divided form, i.e. by forming gas regions inside liquid regions, leading to different kinds of dispersed media...
stabilizers.
Amine oxides are highly polar molecules have a polarity
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
close to that of quaternary ammonium salts. Small amine oxides are very hydrophilic and have an excellent water solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
and a very poor solubility in most organic solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s.
Amine oxides are weak bases
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
with a pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of around 4.5 that form R3N+-OH, cationic hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
s, upon protonation
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
at a pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
below their pKa.
Synthesis
Amine oxides are prepared by oxidation of tertiary amines or pyridine analogs with hydrogen peroxideHydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
(H2O2), Caro's acid or peracids like mCPBA
Meta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling...
in N-oxidation .
Reactions
- Pyrolytic elimination. Amine oxides, when heated to 150 to 200 °C eliminate a hydroxylamine, resulting in an alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. This pyrolyticPyrolysisPyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
syn-elimination reactionElimination reactionAn elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
is known under the name Cope reactionCope reactionThe Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of an amine oxide to form an alkene and a hydroxylamine. The reaction mechanism involves an intramolcular 5-membered cyclic transition state, leading to a syn elimination product...
. The mechanism is similar to that of the Hofmann eliminationHofmann eliminationHofmann elimination is a process where an amine is reacted to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat.After the first step, a quaternary ammonium iodide salt is created...
. - Reduction to amines. Amine oxides are readily converted to the parent amine by common reductionRedoxRedox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reagentReagentA reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s including lithium aluminium hydrideLithium aluminium hydrideLithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, sodium borohydrideSodium borohydrideSodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
, catalytic reduction, zincZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
/ acetic acidAcetic acidAcetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, and ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
/ acetic acid. Pyridine N-oxides can be deoxygenated by phosphorus oxychloride - Sacrificial catalysis. Oxidants can be regenerated by reduction of N-oxides, as in the case of regeneration of osmium tetroxide by N-methylmorpholine oxide.
- O-alkylation. Pyridine N-oxides react with alkyl halides to the O-alkylated product
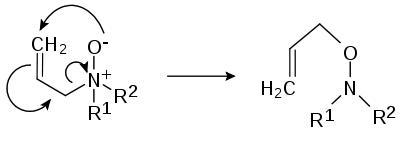
- In the Meisenheimer rearrangement (after Jakob MeisenheimerJakob MeisenheimerJakob Meisenheimer was a German chemist. He made numerous contributions to organic chemistry, the most famous being his proposed structure for a group of compounds now named Meisenheimer complex. He also proposed the mechanism of the Beckmann rearrangement. Later in his career, he reported the...
) certain N-oxides R1R2R3N+O- rearrangeRearrangement reactionA rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
to hydroxylamineHydroxylamineHydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
s R2R3N-O-R1
- in a 1,2-rearrangement:
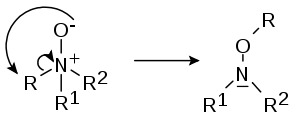
- or a 2,3-rearrangment:
- In the Polonovski reaction a tertiary N-oxide is cleaved by acetic acid anhydride to the corresponding acetamideAcetamideAcetamide is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent...
and aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
:
- In the Polonovski reaction a tertiary N-oxide is cleaved by acetic acid anhydride to the corresponding acetamide
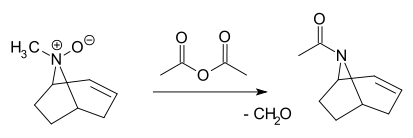
Metabolites
Amine oxides are common metaboliteMetabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
s of medication
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
and psychoactive drug
Psychoactive drug
A psychoactive drug, psychopharmaceutical, or psychotropic is a chemical substance that crosses the blood–brain barrier and acts primarily upon the central nervous system where it affects brain function, resulting in changes in perception, mood, consciousness, cognition, and behavior...
s. Examples include nicotine
Nicotine
Nicotine is an alkaloid found in the nightshade family of plants that constitutes approximately 0.6–3.0% of the dry weight of tobacco, with biosynthesis taking place in the roots and accumulation occurring in the leaves...
, Zolmitriptan
Zolmitriptan
Zolmitriptan is a selective serotonin receptor agonist of the 1B and 1D subtypes. It is a triptan, used in the acute treatment of migraine attacks with or without aura and cluster headaches....
, and morphine
Morphine
Morphine is a potent opiate analgesic medication and is considered to be the prototypical opioid. It was first isolated in 1804 by Friedrich Sertürner, first distributed by same in 1817, and first commercially sold by Merck in 1827, which at the time was a single small chemists' shop. It was more...
.
Amine oxides of anti-cancer drugs
Chemotherapy
Chemotherapy is the treatment of cancer with an antineoplastic drug or with a combination of such drugs into a standardized treatment regimen....
have been developed as prodrug
Prodrug
A prodrug is a pharmacological substance administered in an inactive form. Once administered, the prodrug is metabolised in vivo into an active metabolite, a process termed bioactivation. The rationale behind the use of a prodrug is generally for absorption, distribution, metabolism, and...
s that are metabolized in the oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
deficient cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
tissue
Biological tissue
Tissue is a cellular organizational level intermediate between cells and a complete organism. A tissue is an ensemble of cells, not necessarily identical, but from the same origin, that together carry out a specific function. These are called tissues because of their identical functioning...
to the active drug.
See also
- Functional groupFunctional groupIn organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
- AmineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
, NR3 - HydroxylamineHydroxylamineHydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
, NR2OH - Phosphine oxidePhosphine oxidePhosphine oxides are either inorganic phosphorus compounds such as phosphoryl trichloride or organophosphorus compounds with the formula OPR3, where R = alkyl or aryl...
, PR3=O - SulfoxideSulfoxideA sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
, R2S=O - AzoxyAzoxyAzoxy compounds are a group of chemical compounds sharing a common functional group with the general structure RN=N+R. They are considered N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles. They give 1,3 dipolar cycloaddition with double bonds....
, RN=N+(O–)R RN=N+RO− - TEMPOTEMPOoxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...
(2,2,6,6-Tetramethylpiperidine-1-oxyl), a stable amine oxide radical

