
Hyperconjugation
Encyclopedia

Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, hyperconjugation is the interaction of the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in a sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
(usually C–H or C–C) with an adjacent empty (or partially filled) non-bonding p-orbital or antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
π orbital
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
or filled π orbital, to give an extended molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
that increases the stability of the system. Only electrons in bonds that are β to the positively charged carbon can stabilize a carbocation by hyperconjugation.
History
The term was introduced in 1939 by Robert S. MullikenRobert S. Mulliken
Robert Sanderson Mulliken was an American physicist and chemist, primarily responsible for the early development of molecular orbital theory, i.e. the elaboration of the molecular orbital method of computing the structure of molecules. Dr. Mulliken received the Nobel Prize for chemistry in 1966...
in the course of his work on UV spectroscopy of conjugated molecules. Mulliken observed that on adding alkyl groups to alkenes the spectra shifted to longer wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s. This bathochromic shift
Bathochromic shift
Bathochromic shift is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength ....
is well known in regular conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
compounds such as butadiene. He was also the first to attribute the lower heat of hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
for these substituted compounds (compared to those without substitution) to hyperconjugation. An effect predating the 1939 hyperconjugation concept is the Baker–Nathan effect reported in 1935.
Applications
Hyperconjugation can be used for rationalizing a variety of other chemical phenomena, including the anomeric effectAnomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less hindered equatorial orientation that would...
, the gauche effect, the rotational barrier of ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
, the beta-silicon effect
Beta-silicon effect
The beta-silicon effect also called silicon hyperconjugation in organosilicon chemistry is a special type of hyperconjugation and describes the stabilizing effect of a silicon atom placed in a position one removed from a carbocation. A prerequisite is an antiperiplanar relationship between the two...
, the vibrational frequency of exocyclic carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups, and the relative stability of substituted carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s and substituted carbon centred radicals
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
. Hyperconjugation is proposed by quantum mechanical modeling to be the correct explanation for the preference of the staggered
Staggered
In organic chemistry, a staggered conformation is a chemical conformation of an ethane-like moiety abcX-Ydef in which the substituents a,b,and c are at the maximum distance from d,e,and f...
conformation
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
rather than the old textbook notion of steric hindrance
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
.
Effect on chemical properties
Hyperconjugation affects several properties.- Bond lengthBond length- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
: Hyperconjugation is suggested as a key factor in shortening of sigma bonds (σ bonds). For example, the single C–C bonds in 1,3-butadiene1,3-Butadiene1,3-Butadiene is a simple conjugated diene with the formula C4H6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. When the word butadiene is used, most of the time it refers to 1,3-butadiene....
and methylacetyleneMethylacetyleneMethylacetylene is an alkyne with the chemical formula H3C≡CH. It is a component of MAPP gas along with its isomer 1,2-propadiene , which is commonly used in gas welding...
are approximately 1.46 angstrom in length, much less than the value of around 1.54 Å found in saturated hydrocarbons. This is due mainly to hyperconjugation that gives partial double-bond character of the bond. - Dipole moments: The large increase in dipole moment of 1,1,1-trichloroethane1,1,1-TrichloroethaneThe organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colourless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent...
as compared with chloroformChloroformChloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
can be attributed to hyperconjugated structures. - The heat of formation of molecules with hyperconjugation are greater than sum of their bond energies and the heats of hydrogenation per double bond are less than the heat of hydrogenation of ethyleneEthyleneEthylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
. - Stability of carbocationCarbocationA carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s:
-
- (CH3)3C+ > (CH3)2CH+ > (CH3)CH2+ > CH3+
- The C–C σ bond adjacent to the cation is free to rotate, and, as it does so, the three C–H σ bonds of the methyl group in turn undergoes the stabilization interaction. The more adjacent C-H bonds are the larger hyperconjugation stabilization is.
Hyperconjugation in unsaturated compounds
Early studies in hyperconjugation were performed by Kistiakowsky et al. Their work, first published in 1937, was intended as a preliminary progress report of thermochemical studies of energy changes during addition reactionAddition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
s of various unsaturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
and cyclic compounds. This pioneering work would lead many to investigate the group’s puzzling findings.
Kistiakowsky and fellow researchers collected heats of hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
data during gas-phase reactions of various species containing one double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
. When comparing the monosubstituted alkene compounds propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
, 1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
, 1-heptene
Heptene
Heptene is a higher olefin, or alkene with the formula C7H14.The commercial product is a liquid that is a mixture of isomers. It is used as an additive in lubricants, as a catalyst, and as a surfactant. This chemical is also known as heptylene....
, isopropylethylene, t-butyl ethylene, and neopentylethylene they found that the respective methyl, ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
, n-amyl
Amyl
The word or root amyl has two meanings, in organic chemistry and biochemistry.-Biochemistry:In biochemistry, "amyl" means "pertaining to starch", "amylum" being another word for starch...
, isopropyl
Isopropyl
In organic chemistry, isopropyl is a propyl with a group attached to the secondary carbon. If viewed as a functional group an isopropyl is an organic compound with a propyl group attached at its secondary carbon.The bond is therefore on the middle carbon....
, t-butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
, and neopentyl groups are equally effective in stabilizing the adjacent alkene. The overall range of the ΔH values for these compounds was only 0.8 kcal/mol.
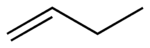 |
||
A portion of Kistiakowsky’s work involved a comparison of other unsaturated compounds in the form of CH2=CH(CH2)n-CH=CH2 (n=0,1,2). These experiments revealed an important result; when n=0, there is an effect of conjugation to the molecule where the ΔH value is lowered by 3.5 kcal. This is likened to the addition of two alkyl groups into ethylene. Kistiakowsky also investigated open chain systems, where the largest value of heat liberated was found to be during the addition to a molecule in the 1,4-position. Cyclic molecules proved to be the most problematic, as it was found that the strain
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
of the molecule would have to be considered. The strain of five-membered rings increased with a decrease degree of unsaturation. This was a surprising result that was further investigated in later work with cyclic acid anhydrides and lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s. Cyclic molecules like benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and its derivatives were also studied, as their behaviors were different from other unsaturated compounds.
Despite the thoroughness of Kistiakowsky’s work, it was not complete and needed further evidence to back up his findings. His work was a crucial first step to the beginnings of the ideas of hyperconjugation and conjugation effects.
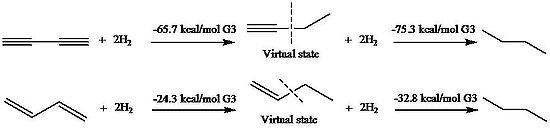
Stabilization of 1,3-butadiyne and 1,3-butadiene
The conjugationConjugation
Conjugation or conjugate may refer to:* Conjugation , the modification of a verb from its basic form* Conjugate , used to rationalize the denominator of a fraction...
of 1,3-butadiene was first evaluated by Kistiakowsky, a conjugative contribution of 3.5 kcal/mol was found based on the energetic comparison of hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
between conjugated species and unconjugated analogues. Rogers et al., who used the method first applied by Kistiakowsky, reported that the conjugation stabilization of 1,3-butadiyne was zero, as the difference of ΔhydH between first and second hydrogenation was zero. The heats of hydrogenation (ΔhydH) were obtained by computational MP2
MP2
MP2 or MP-2 may refer to:* MP 2, an abbreviation for a zone during the Paleocene* MPEG-1 Audio Layer II audio compression format and .mp2 file format...
quantum chemistry method.
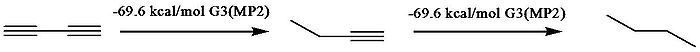
Ab initio
ab initio is a Latin term used in English, meaning from the beginning.ab initio may also refer to:* Ab Initio , a leading ETL Tool Software Company in the field of Data Warehousing.* ab initio quantum chemistry methods...
calculation, which confirmed Rogers’ data. However, they interpreted the data differently by taking into account the hyperconjugation stabilization. To quantify hyperconjugation effect, they designed the following isodesmic reaction
Isodesmic reaction
An isodesmic reaction is a chemical reaction in which the type of chemical bonds broken in the reactant are the same as the type of bonds formed in the reaction product. This type of reaction is often used as a hypothetical reaction in thermochemistry....
s in 1-butyne and 1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
.

1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
, respectively. Employment these virtual states results in a 9.6 kcal/mol conjugative stabilization for 1,3-butadiyne and 8.5 kcal/mol for 1,3-butadiene.
Trends in hyperconjugation
A relatively recent work (2006) by Fernández and Frenking (2006) summarized the trends in hyperconjugation among various groups of acyclic molecules, using energy decomposition analysis or EDA. Fernández and Frenking define this type of analysis as "...a method that uses only the pi orbitals of the interacting fragments in the geometry of the molecule for estimating pi interactions." For this type of analysis, the formation of bonds between various molecular moieties is a combination of three component terms. ΔEelstat represents what Fernández and Frenking call a molecule’s “quasiclassical electrostatic attractions.” The second term, ΔEPauli, represents the molecule’s Pauli repulsion. ΔEorb, the third term, represents stabilizing interactions between orbitals, and is defined as the sum of ΔEpi and ΔEsigma. The total energy of interaction, ΔEint, is the result of the sum of the 3 terms.A group whose ΔEpi values were very thoroughly analyzed were a group of enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s that varied in substituent.
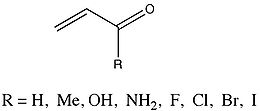
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
, and amino substituents resulted in a decrease in ΔEpi from the parent 2-propenal. Conversely, halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
substituents of increasing atomic mass resulted in increasing ΔEpi. Because both the enone study and Hammett
Hammett equation
The Hammett equation in organic chemistry describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with just two parameters: a substituent constant and a...
analysis study substituent effects (although in different species), Fernández and Frenking felt that comparing the two to investigate possible trends might yield significant insight into their own results. They observed a linear relationship between the ΔEpi values for the substituted enones and the corresponding Hammett constants. The slope of the graph was found to be -51.67, with a correlation coefficient of -0.97 and a standard deviation of 0.54. Fernández and Frenking conclude from this data that ..."the electronic effects of the substituents R on pi conjugation in homo- and heteroconjugated systems is similar and thus appears to be rather independent of the nature of the conjugating system.".
Hyperconjugation: Gronert vs. Schleyer
Gronert (see Gronert model) proposed a 1,3 repulsive interaction, otherwise known as a geminalGeminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
repulsion in place of hyperconjugation. This model explains differences in bond strengths based on differential steric strain relief as a result of bond cleavage. The key point of Gronert's model is that 1,3 repulsions are the major factor in determining stability of C–C of C–H bonds in alkanes. This broad overarching supposition is based on several already existing assumptions:
- The heats of formation of alkanes are determined only by 1,2 bonding interactions and 1,3 repulsive interactions.
- All C–H bonding interactions provide the same stabilization.
- All C–C bonding interactions provide the same stabilization.
- The 1,3 repulsive interactions can be grouped into C–C–C, C–C–H, and H–C–H interactions.
Gronert's work is a logical step from work done 50 years previous by Dunitz, Schomaker, Bauld, Wiberg, Bickelhaupt, Ziegler, and Schleyer. From the results of these groups, Gronert makes a leap of faith to assume that 1,3 repulsive interactions are not uniform and vary in magnitude based on what groups are involved.
Gronert's Method for Evaluating Alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
, Cycloalkane
Cycloalkane
Cycloalkanes are types of alkanes that have one or more rings of carbon atoms in the chemical structure of their molecules. Alkanes are types of organic hydrocarbon compounds that have only single chemical bonds in their chemical structure...
, Alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and Alkyl radical heats of formation:
- ∆Hf = nC–CEC–C + nC=CEC=C + nC–HEC–H + nC–C–CEC–C–C + nC–C–HEC–C–H + nH–C–HEH–C–H – f(C,H)
where
- n = number of each type of interaction or atom
- E = stabilization/destabilization per interaction
- F(C,H) = (170.6 + EC)nC + 52.1nH
- EC = free parameter (correction term for electron pairing in atomic carbon).
The final term converts to heat of formation from values that are fundamentally atomization energies (170.6 kcal/mole for gaseous carbon and 52.1 kcal/mole for hydrogen atoms).
There are several important justifications for Gronert's model:
- Significant geminal repulsion is already expected because groups are separated by less than the combination of their van der Waals radii and there are no bonding interactions. Computational methods also agree that they are important and of the proper magnitude.
- It ís already well-accepted that 1,3 repulsive interactions are important in determining structure.
- Branching has a strong effect on stabilities of alkanes, not just the BDE. No current evidence that differences in bond strengths are controlled only by factors exclusive to the resulting radical. His method addresses the stability of the alkane and alkyl radical.
- Model depends on interactions observed in many systems and affects both structure and reactivity. This is based on the theory that close-range, nonbonded interactions are repulsive, i.e. Steric strain.
Gronert claims that his model successfully reproduces accepted data without invoking hyperconjugation and can perhaps explain well-established trends. However, his analysis involves geminal repulsion absolutely replacing hyperconjugation as a reasonable alternative explanation.
Schleyer's model has several marked differences from Gronert's. He uses a new isodesmic additivity design that in his view faithfully reproduces heats of formation for many alkanes, alkenes, alkynes, and alkyl radicals. All 1,3 interactions are stabilizing so they support branching and hyperconjugation. All adjustable parameters originate from assumption that the magnitude of stabilizations effects at a specific carbon are eased when more than one substituent contributes:
- ∆Hf = base – 2.15n(CH2) – 1,3CCC branching attraction – hyperconjugation
Schleyer notes several advantages of his approach in comparison to Gronert's:
- Gronert's derivation method arbitrarily set some parameters and adjusted the others as best-fit averages of experimental hydrocarbon heats of formation.
- Gronert's derived C–C and C–H bond energy values are higher than those accepted in the literature.
- Gronert uses 7 adjustable parameters, whereas Schleyer uses only 4. Four is the minimum chemically plausible number of parameters, and the added flexibility of additional terms is not necessarily an improvement of general theory.
- Schleyer's single attractive geminal term is sufficient to reproduce data satisfactorily.
- Well-established theories of branching, hyperconjugation and attenuation.
- Schleyer's method depends only on energetic relationships between the simplest hydrocarbon molecules.
Rotational barrier of ethane
An instance where hyperconjugation may be overlooked as a possible chemical explanation is in rationalizing the rotational barrier of ethaneEthane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
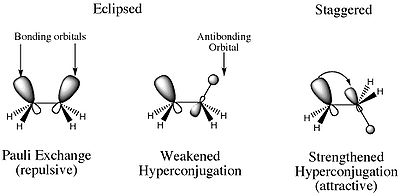
. It had been accepted as early as the 1930s that the staggered conformations of ethane were more stable than the eclipsed conformation. Wilson had proven that the energy barrier between any pair of eclipsed and staggered conformations is approximately 3 kcal/mol, and the generally accepted rationale for this was the unfavorable steric interactions between hydrogen atoms.
In their 2001 paper, however, Pophristic and Goodman revealed that this explanation may be too simplistic. Goodman focused on three principal physical factors: hyperconjugative interactions, exchange repulsion defined by the Pauli exclusion principle
Pauli exclusion principle
The Pauli exclusion principle is the quantum mechanical principle that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles...
, and electrostatic interactions (Coulomb interactions). By comparing a traditional ethane molecule and a hypothetical ethane molecule with all exchange repulsions removed, potential curves were prepared by plotting torsional angle versus energy for each molecule. The analysis of the curves determined that the staggered conformation had no connection to the amount of electrostatic repulsions within the molecule. These results demonstrate that Coulombic forces do not explain the favored staggered conformations, despite the fact that central bond stretching decreases electrostatic interactions.
Goodman also conducted studies to determine the contribution of vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
(between two methyl groups) vs. geminal (between the atoms in a single methyl group) interactions to hyperconjugation. In separate experiments, the geminal and vicinal interactions were removed, and the most stable conformer for each interaction was deduced.
| Deleted interaction | Torsional angle | Corresponding conformer |
|---|---|---|
| None | 60° | Staggered |
| All hyperconjugation | 0° | Eclipsed |
| Vicinal hyperconjugation | 0° | Eclipsed |
| Geminal hyperconjugation | 60° | Staggered |
From these experiments, it can be concluded that hyperconjugative effects delocalize charge and stabilize the molecule. Further, it is the vicinal hyperconjugative effects that keep the molecule in the staggered conformation. Thanks to this work, the following model of the stabilization of the staggered conformation of ethane is now more accepted:
Lewis structure
Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds...
for an ammonium ion indicates a positive charge on the nitrogen atom. In reality, however, the hydrogens are more electropositive than is nitrogen, and thus are the actual carriers of the positive charge. We know this intuitively because bases remove the protons as opposed to the nitrogen atom.
It should be noted that the matter of the rotational barrier of ethane is not settled within the scientific community. An analysis within quantitative molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
shows that 2-orbital-4-electron (steric) repulsions are dominant over hyperconjugation. A valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
study also emphasizes the importance of steric effects.

