
Wittig reaction
Encyclopedia
The Wittig reaction is a chemical reaction
of an aldehyde
or ketone
with a triphenyl phosphonium ylide (often called a Wittig reagent) to give an alkene
and triphenylphosphine oxide
.
 The Wittig reaction was discovered in 1954 by Georg Wittig
The Wittig reaction was discovered in 1954 by Georg Wittig
, for which he was awarded the Nobel Prize in Chemistry
in 1979. It is widely used in organic synthesis
for the preparation of alkenes. It should not be confused with the Wittig rearrangement
.
Wittig reactions are most commonly used to couple aldehydes and ketones to singly substituted phosphine ylide
s. With simple ylides this results in almost exclusively the Z-alkene product. In order to obtain the E-alkene, the Schlosser modification of the Wittig reaction can be performed.
1 influences the stereochemical outcome of nucleophilic addition
to give a predominance of the betaine
3 (c.f. Bürgi–Dunitz angle). Note that for betaine 3 both R1 and R2 as well as PPh3+ and O- are positioned anti (trans-diaxial) to one another.
Carbon-carbon bond rotation gives the betaine 4, which then forms the oxaphosphetane 5. Elimination gives the desired Z-alkene 7 and triphenylphosphine oxide
6. With simple Wittig reagents, the first step occurs easily with both aldehyde
s and ketone
s, and the decomposition of the betaine (to form 5) is the rate-determining step
. However with stabilised ylides (where R1 stabilises the negative charge) the first step is the slowest step, so the overall rate of alkene formation decreases and a bigger proportion of the alkene product is the E-isomer
. This also explains why stabilised reagents fail to react well with sterically hindered ketones.

presented above does not account for all experimental results. Mechanistic studies have been done mostly on unstablilized ylides, because the intermediates can be followed by NMR spectroscopy
. The existence and interconversion of the betaine (3a and 3b) is still under debate and a subject of ongoing research. There is evidence that phosphonium ylides 1 can react with carbonyl compounds 2 via a π²s/π²a [2+2] cycloaddition
to directly form the oxaphosphatanes 4a and 4b. The stereochemistry
of the product 5 is due to the addition of the ylide 1 to the carbonyl 2 and to the ability of the intermediates to equilibrate
. Maryanoff
and Reitz identified the issue about equilibration of Wittig intermediates and termed the process "stereochemical drift". For many years, the stereochemistry of the Wittig reaction, in terms of carbon-carbon bond formation, had been assumed to correspond directly with the Z/E stereochemistry of the alkene products. However, certain reactants do not follow this simple pattern. Lithium
salts can also exert a profound effect on the stereochemical outcome.
 There are distinct differences in the mechanisms of aliphatic and aromatic aldehyde
There are distinct differences in the mechanisms of aliphatic and aromatic aldehyde
s and of aromatic and aliphatic phosphonium ylides. Vedejs et al. have provided evidence that the Wittig reaction of unbranched
aldehydes under lithium-salt-free conditions do not equilibrate and are therefore under kinetic reaction control. Vedejs has put forth a theory to explain the stereoselectivity of stabilized and unstabilized Wittig reactions.
, which is in turn made by the reaction of triphenylphosphine
with an alkyl halide. To form the Wittig reagent (ylide), the phosphonium salt is suspended in a solvent such as diethyl ether
or THF
and treated with a strong base such as phenyllithium
or n-butyllithium
:
The simplest ylide used is methylenetriphenylphosphorane (Ph3P+−C−H2), and it is also a precursor to elaborated Wittig reagents. Alkylation of Ph3P=CH2 with a primary alkyl halide R−CH2−X, produces substituted phosphonium salts:
These salts can be deprotonated
in the usual way to give Ph3P=CH−CH2−R.

The ylide form is a significant contributor, and the carbon is quite nucleophilic
.
More stable phosphoranes are obtained when the ylide contains a group that can stabilise the negative charge from the carbanion
.
For example: Ph3P=CH–COOR, Ph3P=CH–Ph.
These are formed more readily, requiring treatment of the phosphonium salt only with NaOH, and they are usually isolable, crystalline compounds.
These are less reactive than simple ylides, and so they usually fail to react with ketones, necessitating the use of the Horner–Wadsworth–Emmons reaction as an alternative.
They can be prepared from the phosphonium salts using bases weaker than butyllithium, such as alkali metal alkoxide
s and in some cases even sodium hydroxide. They usually give rise to an E-alkene product when they react, rather than the more usual Z-alkene.
synthesis precisely because of its wide applicability. Unlike elimination reaction
s (such as dehydrohalogenation
of alkyl halides), which produce mixtures of alkene regioisomers determined by Saytzeff's rule, the Wittig reaction forms the double bond in one position with no ambiguity.
A large variety of ketone
s and aldehyde
s are effective in the reaction, though carboxylic acid
derivatives such as ester
s fail to react usefully. Thus mono-, di- and trisubstituted alkenes can all be prepared in good yield in most cases. The carbonyl
compound can tolerate several groups such as OH
, OR, aromatic nitro
and even ester groups. There can be a problem with sterically hindered ketones, where the reaction may be slow and give poor yields, particularly with stabilized ylides, and in such cases the Horner–Wadsworth–Emmons (HWE) reaction (using phosphonate esters) is preferred. Another reported limitation is the often labile nature of aldehyde
s which can oxidize, polymerize or decompose. In a so-called Tandem Oxidation-Wittig Process the aldehyde is formed in situ
by oxidation of the corresponding alcohol.
As mentioned above, the Wittig reagent itself is usually derived from a primary alkyl halide, because with most secondary halides the phosphonium salt is formed in poor yield. This means that most tetrasubstituted alkenes are best made by other means. However the Wittig reagent can tolerate many other variants. It may contain alkenes and aromatic rings, and it is compatible with ether
s and even ester
groups. Even C=O and nitrile
groups can be present if conjugated
with the ylide- these are the stabilised ylides mentioned above. Bis-ylides (containing two P=C bonds) have also been made and used successfully.
One limitation relates to the stereochemistry
of the product. With simple ylides, the product is usually mainly the Z-isomer
, although a lesser amount of the E-isomer is often formed also – this is particularly true when ketones are used. If the reaction is performed in DMF
in the presence of LiI
or NaI
, the product is almost exclusively the Z-isomer. If the E-isomer is the desired product, the Schlosser modification may be used. With stabilised ylides the product is mainly the E-isomer, and this same isomer is also usual with the HWE reaction.
 The major limitation of the traditional Wittig reaction is that the reaction goes mainly via the erythro betaine intermediate, which leads to the Z-alkene. However Schlosser & Christmann found that the erythro betaine can be converted to the threo betaine using phenyllithium
The major limitation of the traditional Wittig reaction is that the reaction goes mainly via the erythro betaine intermediate, which leads to the Z-alkene. However Schlosser & Christmann found that the erythro betaine can be converted to the threo betaine using phenyllithium
at low temperature (forming a betaine) followed by HCl
. Upon workup this leads to the E-alkene product as shown.
E. J. Corey and Hisashi Yamamoto found that the utility can be extended to a stereoselective synthesis of allylic alcohols
, by reaction of the betaine ylid with a second aldehyde. For example:
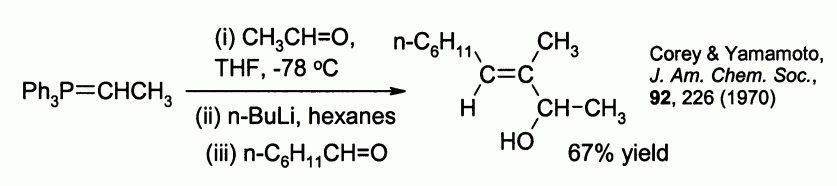
 Because of its reliability and wide applicability, the Wittig reaction has become a standard tool for synthetic organic chemists.
Because of its reliability and wide applicability, the Wittig reaction has become a standard tool for synthetic organic chemists.
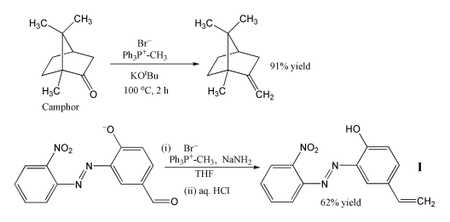
The most popular use of the Wittig reaction is for the introduction of a methylene
group using methylenetriphenylphosphorane (Ph3P=CH2). In the example shown, even a sterically hindered ketone such as camphor
can be successfully converted to its methylene derivative by heating with methyltriphenylphosphonium bromide and potassium tert-butoxide
, which generate the Wittig reagent in situ. In another example, the phosphorane is produced using sodium amide
as a base, and this successfully converts the aldehyde
shown into alkene I in 62% yield. The reaction is performed in cold THF
, and the sensitive nitro
, azo
and phenoxide groups all survive intact. The product can be used to incorporate a photostabiliser into a polymer
, to protect the polymer from damage by UV radiation
.
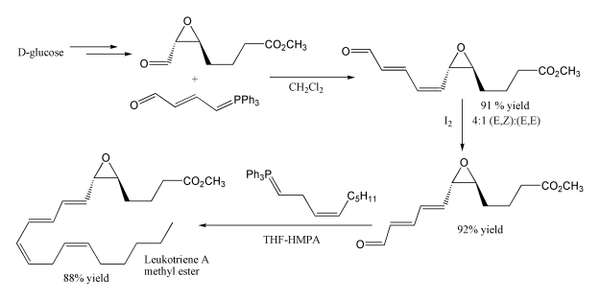
Another example of its use is in the synthesis of leukotriene A
methyl ester. The first step uses a stabilised ylide, where the carbonyl group is conjugated with the ylide preventing self condensation, although unexpectedly this gives mainly the cis product. The second Wittig reaction uses a non-stabilised Wittig reagent, and as expected this gives mainly the cis product. Note that the epoxide
and ester
functional groups survive intact.
 Methoxymethylenetriphenylphosphine
Methoxymethylenetriphenylphosphine
is a Wittig reagent for the homologation of aldehydes.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
with a triphenyl phosphonium ylide (often called a Wittig reagent) to give an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and triphenylphosphine oxide
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
.

Georg Wittig
Georg Wittig was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry with Herbert C...
, for which he was awarded the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 1979. It is widely used in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
for the preparation of alkenes. It should not be confused with the Wittig rearrangement
1,2-Wittig rearrangement
A 1,2-Wittig rearrangement is a categorization of chemical reactions in organic chemistry, and consists of a 1,2-rearrangement of an ether with an alkyllithium compound. The reaction is named for Nobel Prize winning chemist Georg Wittig....
.
Wittig reactions are most commonly used to couple aldehydes and ketones to singly substituted phosphine ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
s. With simple ylides this results in almost exclusively the Z-alkene product. In order to obtain the E-alkene, the Schlosser modification of the Wittig reaction can be performed.
Classical mechanism
The steric bulk of the ylideYlide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
1 influences the stereochemical outcome of nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
to give a predominance of the betaine
Betaines
A betaine in chemistry is any neutral chemical compound with a positively charged cationic functional group such as an quaternary ammonium or phosphonium cation which bears no hydrogen atom and with a negatively charged functional group such as a carboxylate group which may not be adjacent to the...
3 (c.f. Bürgi–Dunitz angle). Note that for betaine 3 both R1 and R2 as well as PPh3+ and O- are positioned anti (trans-diaxial) to one another.
Carbon-carbon bond rotation gives the betaine 4, which then forms the oxaphosphetane 5. Elimination gives the desired Z-alkene 7 and triphenylphosphine oxide
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
6. With simple Wittig reagents, the first step occurs easily with both aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, and the decomposition of the betaine (to form 5) is the rate-determining step
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
. However with stabilised ylides (where R1 stabilises the negative charge) the first step is the slowest step, so the overall rate of alkene formation decreases and a bigger proportion of the alkene product is the E-isomer
Geometric isomerism
In organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
. This also explains why stabilised reagents fail to react well with sterically hindered ketones.

Recent developments
Recent research has shown that the reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
presented above does not account for all experimental results. Mechanistic studies have been done mostly on unstablilized ylides, because the intermediates can be followed by NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
. The existence and interconversion of the betaine (3a and 3b) is still under debate and a subject of ongoing research. There is evidence that phosphonium ylides 1 can react with carbonyl compounds 2 via a π²s/π²a [2+2] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
to directly form the oxaphosphatanes 4a and 4b. The stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of the product 5 is due to the addition of the ylide 1 to the carbonyl 2 and to the ability of the intermediates to equilibrate
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
. Maryanoff
Bruce E. Maryanoff
Bruce Eliot Maryanoff is an American medicinal and organic chemist.-Background and contributions:...
and Reitz identified the issue about equilibration of Wittig intermediates and termed the process "stereochemical drift". For many years, the stereochemistry of the Wittig reaction, in terms of carbon-carbon bond formation, had been assumed to correspond directly with the Z/E stereochemistry of the alkene products. However, certain reactants do not follow this simple pattern. Lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
salts can also exert a profound effect on the stereochemical outcome.

Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and of aromatic and aliphatic phosphonium ylides. Vedejs et al. have provided evidence that the Wittig reaction of unbranched
Branching (chemistry)
In polymer chemistry, branching occurs by the replacement of a substituent, e.g., a hydrogen atom, on a monomer subunit, by another covalently bonded chain of that polymer; or, in the case of a graft copolymer, by a chain of another type...
aldehydes under lithium-salt-free conditions do not equilibrate and are therefore under kinetic reaction control. Vedejs has put forth a theory to explain the stereoselectivity of stabilized and unstabilized Wittig reactions.
Preparation of simple ylides
The Wittig reagent is usually prepared from a phosphonium saltPhosphonium salt
A phosphonium salt is a salt containing the phosphonium ion such as phosphonium iodide . More commonly, phosphonium refers to a quaternary organic derivative such as tetraphenylphosphonium chloride, 4P+ Cl- and tetramethylphosphonium iodide, [P4]+I−.Alkyltriphenylphosphonium salts are widely...
, which is in turn made by the reaction of triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
with an alkyl halide. To form the Wittig reagent (ylide), the phosphonium salt is suspended in a solvent such as diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
or THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
and treated with a strong base such as phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
or n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
:
- Ph3P+−CH2−R X− + C4H9LiN-Butyllithiumn-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
→ Ph3P=CH−R + LiX + C4H10ButaneButane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
The simplest ylide used is methylenetriphenylphosphorane (Ph3P+−C−H2), and it is also a precursor to elaborated Wittig reagents. Alkylation of Ph3P=CH2 with a primary alkyl halide R−CH2−X, produces substituted phosphonium salts:
- Ph3P=CH2 + R-CH2-X → Ph3P+−CH2− CH2−R X−
These salts can be deprotonated
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
in the usual way to give Ph3P=CH−CH2−R.
Structure of the ylide
The Wittig reagent may be written in the phosphorane form (the more familiar representation) or the ylide form:
The ylide form is a significant contributor, and the carbon is quite nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
.
Reactivity
Simple phosphoranes are very reactive and are unstable in the presence of moisture and oxygen in the air. They are therefore prepared in a super dry solvent (usually THF) under nitrogen or argon and the carbonyl compound is added as soon as the phosphorane has been formed.More stable phosphoranes are obtained when the ylide contains a group that can stabilise the negative charge from the carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
.
For example: Ph3P=CH–COOR, Ph3P=CH–Ph.
These are formed more readily, requiring treatment of the phosphonium salt only with NaOH, and they are usually isolable, crystalline compounds.
These are less reactive than simple ylides, and so they usually fail to react with ketones, necessitating the use of the Horner–Wadsworth–Emmons reaction as an alternative.
They can be prepared from the phosphonium salts using bases weaker than butyllithium, such as alkali metal alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
s and in some cases even sodium hydroxide. They usually give rise to an E-alkene product when they react, rather than the more usual Z-alkene.
Scope and limitations
The Wittig reaction has become a popular method for alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
synthesis precisely because of its wide applicability. Unlike elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
s (such as dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
of alkyl halides), which produce mixtures of alkene regioisomers determined by Saytzeff's rule, the Wittig reaction forms the double bond in one position with no ambiguity.
A large variety of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s and aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s are effective in the reaction, though carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
derivatives such as ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s fail to react usefully. Thus mono-, di- and trisubstituted alkenes can all be prepared in good yield in most cases. The carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compound can tolerate several groups such as OH
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
, OR, aromatic nitro
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
and even ester groups. There can be a problem with sterically hindered ketones, where the reaction may be slow and give poor yields, particularly with stabilized ylides, and in such cases the Horner–Wadsworth–Emmons (HWE) reaction (using phosphonate esters) is preferred. Another reported limitation is the often labile nature of aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s which can oxidize, polymerize or decompose. In a so-called Tandem Oxidation-Wittig Process the aldehyde is formed in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
by oxidation of the corresponding alcohol.
As mentioned above, the Wittig reagent itself is usually derived from a primary alkyl halide, because with most secondary halides the phosphonium salt is formed in poor yield. This means that most tetrasubstituted alkenes are best made by other means. However the Wittig reagent can tolerate many other variants. It may contain alkenes and aromatic rings, and it is compatible with ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s and even ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
groups. Even C=O and nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
groups can be present if conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
with the ylide- these are the stabilised ylides mentioned above. Bis-ylides (containing two P=C bonds) have also been made and used successfully.
One limitation relates to the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of the product. With simple ylides, the product is usually mainly the Z-isomer
Geometric isomerism
In organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
, although a lesser amount of the E-isomer is often formed also – this is particularly true when ketones are used. If the reaction is performed in DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
in the presence of LiI
Lithium iodide
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine.-Applications:...
or NaI
Sodium iodide
Sodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
, the product is almost exclusively the Z-isomer. If the E-isomer is the desired product, the Schlosser modification may be used. With stabilised ylides the product is mainly the E-isomer, and this same isomer is also usual with the HWE reaction.
The Schlosser modification

Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
at low temperature (forming a betaine) followed by HCl
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
. Upon workup this leads to the E-alkene product as shown.
E. J. Corey and Hisashi Yamamoto found that the utility can be extended to a stereoselective synthesis of allylic alcohols
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
, by reaction of the betaine ylid with a second aldehyde. For example:

Examples of use

The most popular use of the Wittig reaction is for the introduction of a methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
group using methylenetriphenylphosphorane (Ph3P=CH2). In the example shown, even a sterically hindered ketone such as camphor
Camphor
Camphor is a waxy, white or transparent solid with a strong, aromatic odor. It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel , a large evergreen tree found in Asia and also of Dryobalanops aromatica, a giant of the Bornean forests...
can be successfully converted to its methylene derivative by heating with methyltriphenylphosphonium bromide and potassium tert-butoxide
Potassium tert-butoxide
Potassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
, which generate the Wittig reagent in situ. In another example, the phosphorane is produced using sodium amide
Sodium amide
Sodium amide, commonly called sodamide, is the chemical compound with the formula NaNH2. This solid, which is dangerously reactive toward water, is white when pure, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process...
as a base, and this successfully converts the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
shown into alkene I in 62% yield. The reaction is performed in cold THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
, and the sensitive nitro
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
, azo
Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
and phenoxide groups all survive intact. The product can be used to incorporate a photostabiliser into a polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
, to protect the polymer from damage by UV radiation
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
.
Another example of its use is in the synthesis of leukotriene A
Leukotriene
Leukotrienes are fatty signaling molecules. They were first found in leukocytes . One of their roles is to trigger contractions in the smooth muscles lining the trachea; their overproduction is a major cause of inflammation in asthma and allergic rhinitis...
methyl ester. The first step uses a stabilised ylide, where the carbonyl group is conjugated with the ylide preventing self condensation, although unexpectedly this gives mainly the cis product. The second Wittig reaction uses a non-stabilised Wittig reagent, and as expected this gives mainly the cis product. Note that the epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
and ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
functional groups survive intact.

Methoxymethylenetriphenylphosphine
Methoxymethylenetriphenylphosphine is a Wittig reagent with used as an reagent in the homologization of aldehydes and ketones to extended aldehydes, an organic reaction first reported in 1958 ....
is a Wittig reagent for the homologation of aldehydes.
See also
- Corey–Chaykovsky reagent
- Horner–Wadsworth–Emmons reaction
- Julia–Lythgoe olefination
- Peterson olefinationPeterson olefinationThe Peterson olefination is the chemical reaction of α-silyl carbanions 1 with ketones to form a β-hydroxysilane 2 which eliminates to form alkenes 3.Several reviews have been published....
- Tebbe's reagentTebbe's reagentThe Tebbe reagent is the organometallic compound with the formula 2TiCH2ClAl2. It used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative...
- Organophosphorus chemistry
- Homologation reactionHomologation reactionA homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A Homologous series is a group of compounds that differ by a constant unit, generally a group. The reactants undergo a homologation when the...
External links
- Wittig reaction in Organic SynthesesOrganic SynthesesOrganic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
, Coll. Vol. 10, p. 703 (2004); Vol. 75, p. 153 (1998). (Article) - Wittig reaction in Organic SynthesesOrganic SynthesesOrganic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
, Coll. Vol. 5, p. 361 (1973); Vol. 45, p. 33 (1965). (Article)

