Geometric isomerism
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism
Stereoisomerism
Stereoisomers are isomeric molecules that have the same molecular formula and sequence of bonded atoms , but that differ only in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections...
describing the orientation of functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s within a molecule. In general, such isomers contain double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s, which cannot rotate, but they can also arise from ring structures, wherein the rotation of bonds is greatly restricted. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes.
The terms cis and trans are from Latin, in which cis means "on the same side" and trans means "on the other side" or "across". The term "geometric isomerism" is considered an obsolete synonym of "cis/trans isomerism" by IUPAC. It is sometimes used as a synonym for general stereoisomerism (e.g., optical isomerism being called geometric isomerism); the correct term for non-optical stereoisomerism is diastereomerism.
In organic chemistry
When the substituent groupsSubstituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
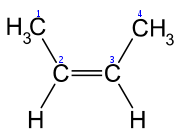
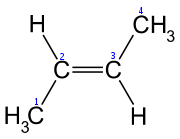
are oriented in the same direction, the diastereomer is referred to as cis, whereas, when the substituents are oriented in opposing directions, the diastereomer is referred to as trans. An example of a small hydrocarbon displaying cis/trans isomerism is 2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
.
Alicyclic compound
Alicyclic compound
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character...
s can also display cis/trans isomerism. As an example of a geometric isomer due to a ring structure, consider 1,2-dichlorocyclohexane:
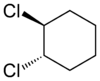 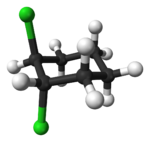 |
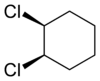 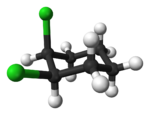 |
| trans-1,2-dichlorocyclohexane | cis-1,2-dichlorocyclohexane |
Comparison of physical properties
Cis and trans isomers often have different physical properties. Differences between isomers, in general, arise from the differences in the shape of the molecule or the overall dipole momentBond dipole moment
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
.
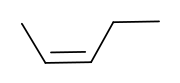 |
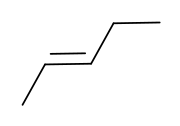 |
| cis-2-pentene | trans-2-pentene |
| cis-1,2-dichloroethene | trans-1,2-dichloroethene |
| cis-butenedioic acid (maleic acid) |
trans-butenedioic acid (fumaric acid) |
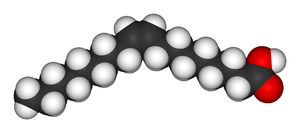 |
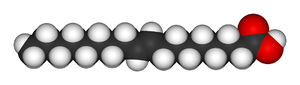 |
| Oleic acid | Elaidic acid |
These differences can be very small, as in the case of the boiling point of straight-chain alkenes, such as 2-pentene
Pentene
Pentene refers to all the alkenes with chemical formula . Each contains one double bond within its molecular structure. There are a total of five different compounds in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure, and whether...
, which is 37°C in the cis isomer and 36°C in the trans isomer. The differences between cis and trans isomers can be larger if polar bonds are present, as in the 1,2-dichloroethene
1,2-Dichloroethene
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a highly flammable, colorless liquid with a sharp, harsh odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene,...
s. The cis isomer in this case has a boiling point of 60.3°C, while the trans isomer has a boiling point of 47.5°C. In the cis isomer the two polar C-Cl bond dipole moment
Bond dipole moment
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
s combine to give an overall molecular dipole, so that there are intermolecular dipole–dipole forces (or Keesom forces) which add to the London dispersion forces and raise the boiling point. In the trans isomer on the other hand, this does not occur because the two C-Cl bond moments cancel and the molecule is non-polar.
The two isomers of butenedioic acid have such large differences in properties and reactivities that they were actually given completely different names. The cis isomer is called maleic acid
Maleic acid
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
and the trans isomer fumaric acid
Fumaric acid
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis...
. Polarity is key in determining relative boiling point as it causes increased intermolecular forces, thereby raising the boiling point. In the same manner, symmetry is key in determining relative melting point as it allows for better packing in the solid state, even if it does not alter the polarity of the molecule. One example of this is the relationship between oleic acid
Oleic acid
Oleic acid is a monounsaturated omega-9 fatty acid found in various animal and vegetable fats. It has the formula CH37CH=CH7COOH. It is an odorless, colourless oil, although commercial samples may be yellowish. The trans isomer of oleic acid is called elaidic acid...
and elaidic acid
Elaidic acid
Elaidic acid is the major trans fat found in hydrogenated vegetable oils and occurs in small amounts in caprine and bovine milk . It is the trans isomer of oleic acid. The name of the elaidinization reaction comes from elaidic acid.Elaidic acid increases CETP activity, which in turn raises VLDL...
; oleic acid, the cis isomer, has a melting point of 13.4 degrees Celsius, making it a liquid at room temperature, while the trans isomer, elaidic acid, has the much higher melting point of 43 degrees Celsius, due to the straighter trans isomer being able to pack more tightly, and is solid at room temperature.
Thus, trans-alkenes, which are less polar and more symmetrical, have lower boiling points and higher melting points, and cis-alkenes, which are generally more polar and less symmetrical, have higher boiling points and lower melting points.
In the case of geometric isomers that are a consequence of double bonds, and, in particular, when both substituents are the same, some general trends usually hold. These trends can be attributed to the fact that the dipoles of the substituents in a cis isomer will add up to give an overall molecular dipole. In a trans isomer, the dipoles of the substituents will cancel out due to their being on opposite site of the molecule. Trans isomers also tend to have lower densities than their cis counterparts.
March observes that trans alkenes tend to have higher melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
s and lower solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
in inert solvents, as trans alkenes, in general, are more symmetrical than cis alkenes.
Vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
coupling constant
J-coupling
J-coupling is the coupling between two nuclear spins due to the influence of bonding electrons on the magnetic field running between the two nuclei. J-coupling contains information about dihedral angles, which can be estimated using the Karplus equation...
s (3JHH), measured by NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, are larger for trans– (range: 12–18 Hz; typical: 15 Hz) than for cis– (range: 0–12 Hz; typical: 8 Hz) isomers.
Stability
Usually, trans isomers are more stable than cis isomers. This is due partly to their shape; the straighter shape of trans isomers leads to hydrogen intermolecular forces that make them more stable . According to Jerry March, trans isomers also have a lower heat of combustionHeat of combustion
The heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
, indicating higher thermochemical
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
stability. In the Benson heat of formation group additivity
Heat of formation group additivity
Heat of formation group additivity methods in thermochemistry enable the calculation and prediction of heat of formation of organic compounds based on additivity. This method was pioneered by S. W...
dataset, cis isomers suffer a 1.10 kcal/mol stability penalty. Exceptions to this rule exist, such as 1,2-difluoroethylene, 1,2-difluorodiazene (FN=NF), and several other halogen- and oxygen-substituted ethylenes. In these cases, the cis isomer is more stable than the trans isomer. This phenomenon is called the cis effect.
E/Z notation
-1-bromo-1,2-dicholroethene.svg.png)
Whether a molecular configuration is designated E or Z is determined by the Cahn-Ingold-Prelog priority rule
Cahn-Ingold-Prelog priority rule
The Cahn–Ingold–Prelog priority rules, CIP system or CIP conventions are a set of rules used in organic chemistry to name the stereoisomers of a molecule. A molecule may contain any number of stereocenters and any number of double bonds, and each gives rise to two possible configurations...
s; higher atomic numbers are given higher priority. For each of the two atoms in the double bond, it is necessary to determine the priority of each substituent. If both the higher-priority substituents are on the same side, the arrangement is Z; if on opposite sides, the arrangement is E.
Inorganic coordination complexes
In inorganic coordination complexes with octahedral or square planar geometries, there are also cis isomers in which similar ligands are closer together and trans isomers in which they are further apart.For example, there are two isomers of square planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
Pt(NH3)2Cl2, as explained by Alfred Werner
Alfred Werner
Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry...
in 1893. The cis isomer, whose full name is cis-diamminedichloroplatinum(II), was shown in 1969 by Barnett Rosenberg
Barnett Rosenberg
Barnett Rosenberg was an American chemist best known for the discovery of the anti-cancer drug cisplatin.Rosenberg graduated from Brooklyn College in 1948 and obtained his PhD in Physics at New York University in 1956...
to have antitumor activity, and is now a chemotherapy drug known by the short name cisplatin
Cisplatin
Cisplatin, cisplatinum, or cis-diamminedichloroplatinum is a chemotherapy drug. It is used to treat various types of cancers, including sarcomas, some carcinomas , lymphomas, and germ cell tumors...
. In contrast, the trans isomer (transplatin) has no useful anticancer activity. Each isomer can be synthesized using the trans effect
Trans effect
In inorganic chemistry, the trans effect is the labilization of ligands that are trans to certain other ligands, which can thus be regarded as trans-directing ligands...
to control which isomer is produced.
For octahedral complexes of formula MX4Y2, two isomers also exist. (Here M is a metal atom, and X and Y are two different types of ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s.) In the cis isomer, the two Y ligands are adjacent to each other at 90°, as is true for the two chlorine atoms shown in green in cis-[Co(NH3)4Cl2]+, at left. In the trans isomer shown at right, the two Cl atoms are on opposite sides of the central Co atom.
A related type of isomerism in octahedral MX3Y3 complexes is
facial-meridional (or fac/mer) isomerism, in which different numbers of ligands are cis or trans to each other.
See also
- E-Z notationE-Z notationE-Z notation, or the E-Z convention, is the IUPAC preferred method of describing the stereochemistry of double bonds in organic chemistry...
- IsomerIsomerIn chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
- Structural isomerismStructural isomerismStructural isomerism, or constitutional isomerism , is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders, as opposed to stereoisomerism. There are multiple synonyms for constitutional isomers.Three categories of constitutional isomers...
- Chirality (chemistry)Chirality (chemistry)A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
- Trans fatTrans fatTrans fat is the common name for unsaturated fat with trans-isomer fatty acid. Because the term refers to the configuration of a double carbon-carbon bond, trans fats are sometimes monounsaturated or polyunsaturated, but never saturated....

