Ylide
Encyclopedia
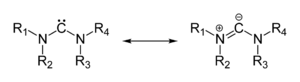
An ylide or ylid is a neutral
dipolar
molecule
containing a formally negatively charged atom
(usually a carbanion
) directly attached to a hetero atom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compound
s. They appear in organic chemistry
as reagent
s or reactive intermediate
s.
The class name "ylide" for the compound should not be confused with the suffix
"-ylide". The name "ylide" derives from the negatively charged carbon
or alkyl moiety in the molecule which is given the "ide" suffix that denotes a negatively charged unit (c.f. chloride, oxide, nitride).
The actual electron distribution in the molecules and hence the relative importance of the ylide and ylene forms is dependent on the "onium" center and substituent pattern (the identity of the various R groups).
for double bond
synthesis from carbonyl groups (C=O). The positive charge in these Wittig reagents is carried by a phosphorus
atom with three phenyl substituents and one bond to a carbon
bearing a negative charge and two substituents, commonly alkyl groups. Ylids can be 'stabilised' or 'non-stabilised'. Non-stabilised ylids react readily with both aldehyde
s and ketone
s whereas stabilised will only react with aldehydes . Stabilised ylids react with both aldehydes and ketones very rapidly in the HWE reaction.
A phosphonium ylide can be prepared rather straightforwardly. Typically, a phosphine
(e.g. triphenylphosphine
) is allowed to react with an alkyl halide in a mechanism analogous to that of an SN2 reaction
. This forms an alkyltriphenylphosphonium salt which is then allowed to react with a strong base (in this case, dimsyl sodium) to form the ylide.
The salt products are not shown. Also, the product shown here is shown in the ylide form; however, it could also be shown as the phosphorane form in which the bond to phosphorus is a double bond with the methylene group. Due to an inductive effect, the trio of phenyl groups allows phosphorus to bear such a buildup of positive charge and shifts the negative charge to carbon, creating a reactive species.
Due to the SN2 mechanism, a less sterically hindered alkyl halide reacts more favorably with triphenylphosphine than an alkyl halide with significant steric hindrance (such as tert-butyl bromide
). Because of this, there will typically be one synthetic route in a synthesis involving such compounds that is more favorable than another.
s or in the Stevens rearrangement
.
s. Oxonium ylids (RR'-O+-C-R'R) are prepared by reaction of ethers with diazo
compounds.
-based ylids also exist such as azomethine ylids with the general structure:
These compounds can be envisioned as iminium
cations placed next to a carbanion
. The substituent
s R1, R2 are electron withdrawing groups. These ylids can be generated by condensation of an α-amino acid
and an aldehyde
or by thermal ring opening reaction of certain N-substituted aziridine
s. Stable carbenes also have a ylidic resonance contributor e.g.:
s. After a [2,3]-rearrangement a homoallylhalide is obtained.
The active form of Tebbe's reagent
is often considered a titanium ylide. Like the Wittig reagent, it is able to replace the oxygen atom on carbonyl groups with a methylene group. Compared with the Wittig reagent, it has more functional group tolerance.
(for phosphorus) but there are more.
s and interact in 1,3-dipolar cycloaddition
s. For instance an azomethine ylide is a dipole in the Prato reaction
with fullerene
s.
s. The Sommelet-Hauser rearrangement
is an example of a [2,3]-sigmatropic reaction. The Stevens rearrangement
is a [1,2]-rearrangement.
A[3,3] -sigmatropic reaction
has been observed in certain phosphonium ylids
:
The initial addition reaction is followed by an elimination reaction
.
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
dipolar
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
containing a formally negatively charged atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
(usually a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
) directly attached to a hetero atom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compound
Dipolar compound
In organic chemistry, a dipolar compound or simply dipole is an electrically neutral molecule carrying a positive and a negative charge in at least one canonical description. In most dipolar compounds the charges are delocalized....
s. They appear in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
as reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s or reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s.
The class name "ylide" for the compound should not be confused with the suffix
Suffix
In linguistics, a suffix is an affix which is placed after the stem of a word. Common examples are case endings, which indicate the grammatical case of nouns or adjectives, and verb endings, which form the conjugation of verbs...
"-ylide". The name "ylide" derives from the negatively charged carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
or alkyl moiety in the molecule which is given the "ide" suffix that denotes a negatively charged unit (c.f. chloride, oxide, nitride).
Resonance structures
Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form:The actual electron distribution in the molecules and hence the relative importance of the ylide and ylene forms is dependent on the "onium" center and substituent pattern (the identity of the various R groups).
Phosphonium ylides
Phosphonium ylides are used in the Wittig reactionWittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
for double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
synthesis from carbonyl groups (C=O). The positive charge in these Wittig reagents is carried by a phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
atom with three phenyl substituents and one bond to a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
bearing a negative charge and two substituents, commonly alkyl groups. Ylids can be 'stabilised' or 'non-stabilised'. Non-stabilised ylids react readily with both aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s whereas stabilised will only react with aldehydes . Stabilised ylids react with both aldehydes and ketones very rapidly in the HWE reaction.
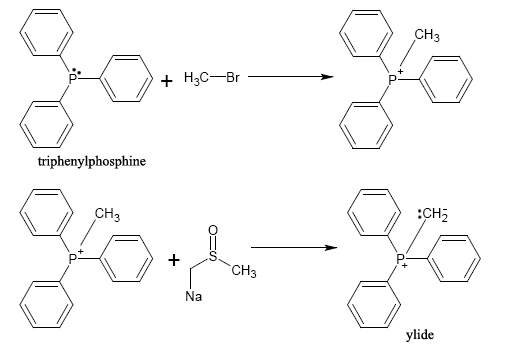
A phosphonium ylide can be prepared rather straightforwardly. Typically, a phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
(e.g. triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
) is allowed to react with an alkyl halide in a mechanism analogous to that of an SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
. This forms an alkyltriphenylphosphonium salt which is then allowed to react with a strong base (in this case, dimsyl sodium) to form the ylide.

The salt products are not shown. Also, the product shown here is shown in the ylide form; however, it could also be shown as the phosphorane form in which the bond to phosphorus is a double bond with the methylene group. Due to an inductive effect, the trio of phenyl groups allows phosphorus to bear such a buildup of positive charge and shifts the negative charge to carbon, creating a reactive species.
Due to the SN2 mechanism, a less sterically hindered alkyl halide reacts more favorably with triphenylphosphine than an alkyl halide with significant steric hindrance (such as tert-butyl bromide
Tert-Butyl bromide
tert-Butyl bromide is an organic compound with a tert-butyl carbon frame and a bromine substituent. This organobromine compound is used as a raw material in synthetic organic chemistry. The compound is isomeric with 1-bromobutane and 2-bromobutane....
). Because of this, there will typically be one synthetic route in a synthesis involving such compounds that is more favorable than another.
Based on sulfur
Other common ylids include sulfonium ylids and sulfoxonium ylids, for instance the Corey-Chaykovsky reagent used in the preparation of epoxideEpoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s or in the Stevens rearrangement
Stevens rearrangement
The Stevens rearrangement in organic chemistry is an organic reaction converting quaternary ammonium salts and sulfonium salts to the corresponding amines or sulfides in presence of a strong base in a 1,2-rearrangement....
.
Based on oxygen
Carbonyl ylides (RR'C=O+C-RR') can form by ring-opening of epoxideEpoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s. Oxonium ylids (RR'-O+-C-R'R) are prepared by reaction of ethers with diazo
Diazo
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
compounds.
Based on nitrogen
Certain nitrogenNitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
-based ylids also exist such as azomethine ylids with the general structure:
These compounds can be envisioned as iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
cations placed next to a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
. The substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s R1, R2 are electron withdrawing groups. These ylids can be generated by condensation of an α-amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or by thermal ring opening reaction of certain N-substituted aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
s. Stable carbenes also have a ylidic resonance contributor e.g.:
Other
Halonium ylides can be prepared from allyl halides and metal carbenoidCarbenoid
In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of...
s. After a [2,3]-rearrangement a homoallylhalide is obtained.
The active form of Tebbe's reagent
Tebbe's reagent
The Tebbe reagent is the organometallic compound with the formula 2TiCH2ClAl2. It used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative...
is often considered a titanium ylide. Like the Wittig reagent, it is able to replace the oxygen atom on carbonyl groups with a methylene group. Compared with the Wittig reagent, it has more functional group tolerance.
Ylide reactions
An important ylide reaction is of course the Wittig reactionWittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
(for phosphorus) but there are more.
dipolar cycloadditions
Some ylids are 1,3-dipole1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms...
s and interact in 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
s. For instance an azomethine ylide is a dipole in the Prato reaction
Prato reaction
The Prato reaction in fullerene chemistry describes the functionalization of fullerenes and nanotubes with azomethine ylides in a 1,3-dipolar cycloaddition...
with fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
s.
Sigmatropic rearrangements
Many ylids react in sigmatropic reactionSigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon-carbon...
s. The Sommelet-Hauser rearrangement
Sommelet-Hauser rearrangement
The Sommelet–Hauser rearrangement is a rearrangement reaction of certain benzyl quaternary ammonium salts...
is an example of a [2,3]-sigmatropic reaction. The Stevens rearrangement
Stevens rearrangement
The Stevens rearrangement in organic chemistry is an organic reaction converting quaternary ammonium salts and sulfonium salts to the corresponding amines or sulfides in presence of a strong base in a 1,2-rearrangement....
is a [1,2]-rearrangement.
A
Sigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon-carbon...
has been observed in certain phosphonium ylids
Allylic rearrangements
Wittig reagents are found to react as nucleophiles in SN2' substitutionAllylic rearrangement
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution....
:
The initial addition reaction is followed by an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
.
See also
- 1,3-dipole1,3-dipoleIn organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms...
- ZwitterionZwitterionIn chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
: a neutral molecule with one or more pairs of positive and negative charges - Betaine: a neutral molecule with an oniumOnium compoundsOnium compounds are cations derived by the protonation of mononuclear parent hydrides of elements of the nitrogen group , chalcogens , or halogens , and similar cations derived by the substitution of hydrogen atoms in the former by other groups, such as organic radicals, or halogens, for example...
cation and a negative charge