History of the periodic table
Encyclopedia
The history of the periodic table reflects over a century of growth in the understanding of chemical properties, and culminates with the publication of the first actual periodic table
by Dmitri Mendeleev
in 1869. While Mendeleev built upon earlier discoveries by such scientists as Antoine-Laurent de Lavoisier and Stanislao Cannizzaro
, the Russian scientist is generally given sole credit for development of the actual periodic table
itself.
The table itself is a visual representation of the periodic law which states that certain properties of element
s repeat periodically when arranged by atomic number
. The table arranges elements into vertical columns (groups) and horizontal rows (periods) to display these commonalities.
s like gold
, silver
and copper
from antiquity, as these can all be discovered in nature in native form and are relatively simple to mine with primitive tools. However, the notion that there were a limited number of elements from which everything was composed originated with the Greek philosopher Aristotle
. About 330 B.C Aristotle proposed that everything is made up of a mixture of one or more of four "roots" (originally put forth by the Sicilian
philosopher Empedocles
), but later renamed elements
by Plato. The four elements were earth
, water
, air
and fire
. While the concept of an element was thus introduced, Aristotle's and Plato's ideas did nothing to advance the understanding of the nature of matter.
was the first person recorded to have discovered a new element. Brand was a bankrupt German merchant who was trying to discover the Philosopher's Stone
— a mythical object that was supposed to turn inexpensive base metal
s into gold. He experimented with distilling human urine until in 1649 he finally obtained a glowing white substance which he named phosphorus
. He kept his discovery secret, until 1680 when Robert Boyle
rediscovered it and it became public. This and related discoveries raised the question of what it means for a substance to be an "element".
In 1661 Boyle defined an element as a substance that cannot be broken down into a simpler substance by a chemical reaction. This simple definition actually served for nearly 300 years (until the development of the notion of subatomic particles), and even today is taught in introductory chemistry classes.
 Lavoisier
Lavoisier
's Traité Élémentaire de Chimie
(Elementary Treatise of Chemistry, 1789, translated into English by Robert Kerr
) is considered to be the first modern chemical textbook
. It contained a list of elements, or substances that could not be broken down further, which included oxygen
, nitrogen
, hydrogen
, phosphorus
, mercury
, zinc
, and sulfur
. It also forms the basis for the modern list of elements. His list, however, also included light
and caloric
, which he believed to be material substances. While many leading chemists of the time refused to believe Lavoisier's new revelations, the Elementary Treatise was written well enough to convince the younger generation. However, as Lavoisier's descriptions only classified elements as metals and non-metals, it fell short of a complete analysis.
began to formulate one of the earliest attempts to classify the elements. He found that some elements formed groups of three with related properties. He termed these groups "triads
".
Some triads classified by Döbereiner are:
In all of the triads, the atomic weight of the second element was almost exactly the average of the atomic weights of the first and third element.
, a French geologist, was the first person to notice the periodicity of the elements — similar elements seem to occur at regular intervals when they are ordered by their atomic weights. He devised an early form of periodic table, which he called the telluric helix. With the elements arranged in a spiral on a cylinder by order of increasing atomic weight, de Chancourtois saw that elements with similar properties lined up vertically. His chart included some ions and compounds in addition to elements. His paper was published in 1862, but used geological rather than chemical terms and did not include a diagram; as a result, it received little attention until the work of Dimitri Mendeleev.
was an English chemist who in 1865 classified the 56 elements that had been discovered at the time into eleven groups which were based on similar physical properties.
Newlands noted that many pairs of similar elements existed which differed by some multiple of eight in atomic weight. However, his law of octaves, likening this periodicity of eights to the musical scale, was ridiculed by his contemporaries. It was not until the following century, with Gilbert N. Lewis
' valence bond theory
(1916) and Irving Langmuir
's octet theory of chemical bonding
(1919) that the importance of the periodicity of eight would be accepted.
much like the one we use today. Mendeleev arranged the elements in a table ordered by atomic weight
, corresponding to relative molar mass
as defined today. It is sometimes said that he played "chemical solitaire" on long train journeys using cards with various facts of known elements. On March 6, 1869, a formal presentation was made to the Russian Chemical Society, entitled The Dependence Between the Properties of the Atomic Weights of the Elements. His table was published in an obscure Russian journal but quickly republished in a German journal, Zeitschrift für Chemie (Eng., "Chemistry Magazine"), in 1869. It stated:
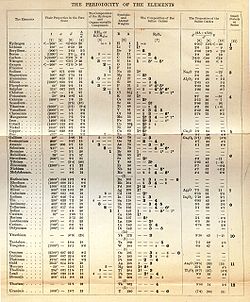 Scientific benefits of Mendeleev's table
Scientific benefits of Mendeleev's table
Shortcomings of Mendeleev's table
, but by valence
alone. Finally, Meyer never came to the idea of predicting new elements and correcting atomic weights. Only a few months after Mendeleev published his periodic table of all known elements (and predicted some new elements to complete the table, plus some corrected atomic weights), Meyer published a virtually identical table. While a few people consider Meyer and Mendeleev the co-creators of the periodic table, most agree that, by itself, Mendeleev's accurate prediction of the qualities of the undiscovered elements lands him the larger share of credit. In any case, at the time Mendeleev's predictions greatly impressed his contemporaries and were eventually found to be correct. An English chemist, William Odling
, also drew up a table that is remarkably similar to that of Mendeleev, in 1864.
found a relationship between an element's X-ray
wavelength and its atomic number
(Z), and therefore resequenced the table by nuclear charge rather than atomic weight. Before this discovery, atomic numbers were just sequential numbers based on an element's atomic weight. Moseley's discovery showed that atomic numbers had an experimentally measurable basis.
Thus Moseley placed argon
(Z=18) before potassium
(Z=19) based on their X-ray wavelengths, despite the fact that argon has a greater atomic weight (39.9) than potassium (39.1). The new order agrees with the chemical properties of these elements, since argon is a noble gas
and potassium an alkali metal
. Similarly, Moseley placed cobalt
before nickel
, and was able to explain that tellurium occurs before iodine
without revising the experimental atomic weight of tellurium (127.6) as proposed by Mendeleev.
Moseley's research also showed that there were gaps in his table at atomic numbers 43 and 61 which are now known to be Technetium
and Promethium
, respectively, both radioactive and not naturally occurring. Following in the footsteps of Dmitri Mendeleev, Henry Moseley also predicted new elements.
experienced unexpected difficulty isolating Americium
(95) and Curium
(96). He began wondering if these elements more properly belonged to a different series which would explain why the expected chemical properties of the new elements were different. In 1945, he went against the advice of colleagues and proposed a significant change to Mendeleev's table: the actinide series.
Seaborg's actinide concept
of heavy element electronic structure, predicting that the actinides form a transition series analogous to the rare earth series of lanthanide elements, is now well accepted in the scientific community and included in all standard configurations of the periodic table. The actinide series are the second row of the f-block (5f series) and comprise the elements from Actinium to Lawrencium. Seaborg's subsequent elaborations of the actinide concept theorized a series of superheavy elements in a transactinide series comprising elements 104 through 121 and a superactinide series inclusive of elements 122 through 153.
IUPAC suggest five "main discovery periods":
In 1998, a 35-by-65 foot periodic table was constructed at the Science Museum of Virginia and is a Guinness World Record
.
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
by Dmitri Mendeleev
Dmitri Mendeleev
Dmitri Ivanovich Mendeleev , was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements...
in 1869. While Mendeleev built upon earlier discoveries by such scientists as Antoine-Laurent de Lavoisier and Stanislao Cannizzaro
Stanislao Cannizzaro
Stanislao Cannizzaro, FRS was an Italian chemist. He is remembered today largely for the Cannizzaro reaction and for his influential role in the atomic-weight deliberations of the Karlsruhe Congress in 1860.-Biography:...
, the Russian scientist is generally given sole credit for development of the actual periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
itself.
The table itself is a visual representation of the periodic law which states that certain properties of element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s repeat periodically when arranged by atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
. The table arranges elements into vertical columns (groups) and horizontal rows (periods) to display these commonalities.
Elemental ideas from ancient times
People have known about some chemical elementChemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s like gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
, silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
from antiquity, as these can all be discovered in nature in native form and are relatively simple to mine with primitive tools. However, the notion that there were a limited number of elements from which everything was composed originated with the Greek philosopher Aristotle
Aristotle
Aristotle was a Greek philosopher and polymath, a student of Plato and teacher of Alexander the Great. His writings cover many subjects, including physics, metaphysics, poetry, theater, music, logic, rhetoric, linguistics, politics, government, ethics, biology, and zoology...
. About 330 B.C Aristotle proposed that everything is made up of a mixture of one or more of four "roots" (originally put forth by the Sicilian
Sicily
Sicily is a region of Italy, and is the largest island in the Mediterranean Sea. Along with the surrounding minor islands, it constitutes an autonomous region of Italy, the Regione Autonoma Siciliana Sicily has a rich and unique culture, especially with regard to the arts, music, literature,...
philosopher Empedocles
Empedocles
Empedocles was a Greek pre-Socratic philosopher and a citizen of Agrigentum, a Greek city in Sicily. Empedocles' philosophy is best known for being the originator of the cosmogenic theory of the four Classical elements...
), but later renamed elements
Classical element
Many philosophies and worldviews have a set of classical elements believed to reflect the simplest essential parts and principles of which anything consists or upon which the constitution and fundamental powers of anything are based. Most frequently, classical elements refer to ancient beliefs...
by Plato. The four elements were earth
Earth (classical element)
Earth, home and origin of humanity, has often been worshipped in its own right with its own unique spiritual tradition.-European tradition:Earth is one of the four classical elements in ancient Greek philosophy and science. It was commonly associated with qualities of heaviness, matter and the...
, water
Water (classical element)
Water is one of the elements in ancient Greek philosophy, in the Asian Indian system Panchamahabhuta, and in the Chinese cosmological and physiological system Wu Xing...
, air
Air (classical element)
Air is often seen as a universal power or pure substance. Its supposed fundamental importance to life can be seen in words such as aspire, inspire, perspire and spirit, all derived from the Latin spirare.-Greek and Roman tradition:...
and fire
Fire (classical element)
Fire has been an important part of all cultures and religions from pre-history to modern day and was vital to the development of civilization. It has been regarded in many different contexts throughout history, but especially as a metaphysical constant of the world.-Greek and Roman tradition:Fire...
. While the concept of an element was thus introduced, Aristotle's and Plato's ideas did nothing to advance the understanding of the nature of matter.
Age of Enlightenment
Hennig BrandHennig Brand
Hennig Brand was a merchant and alchemist in Hamburg, Germany. He discovered phosphorus around 1669.-Early life:The circumstances of Brand's birth are unknown. Some sources describe his origins as humble and indicate that he had been an apprentice glass-maker as a young man...
was the first person recorded to have discovered a new element. Brand was a bankrupt German merchant who was trying to discover the Philosopher's Stone
Philosopher's stone
The philosopher's stone is a legendary alchemical substance said to be capable of turning base metals into gold or silver. It was also sometimes believed to be an elixir of life, useful for rejuvenation and possibly for achieving immortality. For many centuries, it was the most sought-after goal...
— a mythical object that was supposed to turn inexpensive base metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s into gold. He experimented with distilling human urine until in 1649 he finally obtained a glowing white substance which he named phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
. He kept his discovery secret, until 1680 when Robert Boyle
Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
rediscovered it and it became public. This and related discoveries raised the question of what it means for a substance to be an "element".
In 1661 Boyle defined an element as a substance that cannot be broken down into a simpler substance by a chemical reaction. This simple definition actually served for nearly 300 years (until the development of the notion of subatomic particles), and even today is taught in introductory chemistry classes.
Antoine-Laurent de Lavoisier

Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
's Traité Élémentaire de Chimie
Traité Élémentaire de Chimie
Traité élémentaire de chimie is an influential textbook written by Antoine Lavoisier published in 1789 and translated into English by Robert Kerr in 1790.The book is considered to be the first modern chemical textbook...
(Elementary Treatise of Chemistry, 1789, translated into English by Robert Kerr
Robert Kerr (writer)
Robert Kerr FRS was a scientific writer and translator from Scotland.Kerr was born in Roxburghshire as the son of a jeweller. He studied medicine at the University of Edinburgh and practised at the Edinburgh Foundling Hospital as a surgeon...
) is considered to be the first modern chemical textbook
Textbook
A textbook or coursebook is a manual of instruction in any branch of study. Textbooks are produced according to the demands of educational institutions...
. It contained a list of elements, or substances that could not be broken down further, which included oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. It also forms the basis for the modern list of elements. His list, however, also included light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
and caloric
Caloric theory
The caloric theory is an obsolete scientific theory that heat consists of a self-repellent fluid called caloric that flows from hotter bodies to colder bodies. Caloric was also thought of as a weightless gas that could pass in and out of pores in solids and liquids...
, which he believed to be material substances. While many leading chemists of the time refused to believe Lavoisier's new revelations, the Elementary Treatise was written well enough to convince the younger generation. However, as Lavoisier's descriptions only classified elements as metals and non-metals, it fell short of a complete analysis.
Johann Wolfgang Döbereiner
In 1817, Johann Wolfgang DöbereinerJohann Wolfgang Döbereiner
Johann Wolfgang Döbereiner was a German chemist who is best known for work that foreshadowed the periodic law for the chemical elements.- Life and work :...
began to formulate one of the earliest attempts to classify the elements. He found that some elements formed groups of three with related properties. He termed these groups "triads
Dobereiner's Triads
Before the creation of the periodic table by Mendeleev, there were several laws for the classification of the elements. One of such laws was, Dobereiner's Law of Triads...
".
Some triads classified by Döbereiner are:
- chlorineChlorineChlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, bromineBromineBromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
, and iodineIodineIodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... - calciumCalciumCalcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
, strontiumStrontiumStrontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
, and bariumBariumBarium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with... - sulfurSulfurSulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, seleniumSeleniumSelenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
, and tellurium - lithiumLithiumLithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
, sodiumSodiumSodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, and potassiumPotassiumPotassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
In all of the triads, the atomic weight of the second element was almost exactly the average of the atomic weights of the first and third element.
Classifying Elements
By 1869, a total of 63 elements had been discovered. As the number of known elements grew, scientists began to recognize patterns in the way chemicals reacted and began to devise ways to classify the elements.Alexandre-Emile Béguyer de Chancourtois
Alexandre-Emile Béguyer de ChancourtoisAlexandre-Emile Béguyer de Chancourtois
Alexandre-Emile Béguyer de Chancourtois was a French geologist and mineralogist who was the first to arrange the chemical elements in order of atomic weights, doing so in 1862. De Chancourtois only published his paper, but did not publish his actual graph with the proposed arrangement...
, a French geologist, was the first person to notice the periodicity of the elements — similar elements seem to occur at regular intervals when they are ordered by their atomic weights. He devised an early form of periodic table, which he called the telluric helix. With the elements arranged in a spiral on a cylinder by order of increasing atomic weight, de Chancourtois saw that elements with similar properties lined up vertically. His chart included some ions and compounds in addition to elements. His paper was published in 1862, but used geological rather than chemical terms and did not include a diagram; as a result, it received little attention until the work of Dimitri Mendeleev.
John Newlands
John NewlandsJohn Alexander Reina Newlands
John Alexander Reina Newlands was an English chemist who invented the Periodic Table.Newlands was born in London and was the son of a scottish Presbyterian minister and his Italian wife....
was an English chemist who in 1865 classified the 56 elements that had been discovered at the time into eleven groups which were based on similar physical properties.
Newlands noted that many pairs of similar elements existed which differed by some multiple of eight in atomic weight. However, his law of octaves, likening this periodicity of eights to the musical scale, was ridiculed by his contemporaries. It was not until the following century, with Gilbert N. Lewis
Gilbert N. Lewis
Gilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and...
' valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
(1916) and Irving Langmuir
Irving Langmuir
Irving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his...
's octet theory of chemical bonding
(1919) that the importance of the periodicity of eight would be accepted.
Dimitri Mendeleev
Dimitri Mendeleev, a Russian chemist, was the first scientist to make a periodic tablePeriodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
much like the one we use today. Mendeleev arranged the elements in a table ordered by atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
, corresponding to relative molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
as defined today. It is sometimes said that he played "chemical solitaire" on long train journeys using cards with various facts of known elements. On March 6, 1869, a formal presentation was made to the Russian Chemical Society, entitled The Dependence Between the Properties of the Atomic Weights of the Elements. His table was published in an obscure Russian journal but quickly republished in a German journal, Zeitschrift für Chemie (Eng., "Chemistry Magazine"), in 1869. It stated:
- The elementsChemical elementA chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
, if arranged according to their atomic weightAtomic weightAtomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
s, exhibit an apparent periodicity of properties. - Elements which are similar as regards to their chemical properties have atomic weights which are either of nearly the same value (e.g., Pt, Ir, Os) or which increase regularly (e.g., K, Rb, Cs).
- The arrangement of the elements, or of groups of elements in the order of their atomic weights, corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, Ba, C, N, O, and Sn.
- The elements which are the most widely diffused have small atomic weights.
- The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule determines the character of a compound body.
- We must expect the discovery of many yet unknown elements–for example, elements analogous to aluminiumAluminiumAluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
and siliconSiliconSilicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
–whose atomic weight would be between 65 and 75. - The atomic weight of an element may sometimes be amended by a knowledge of those of its contiguous elements. Thus the atomic weight of tellurium must lie between 123 and 126, and cannot be 128. (This was based on the position of tellurium between antimonyAntimonyAntimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
and iodineIodineIodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
whose atomic weight is 127. However Moseley later explained the position of these elements without revising the atomic weight values — see below.) - Certain characteristic properties of elements can be foretold from their atomic weights.

- Mendeleev predicted the discovery of other elementsMendeleev's predicted elementsProfessor Dmitri Mendeleev published the first Periodic Table of the Atomic Elements in 1869 based on properties which appeared with some regularity as he laid out the elements from lightest to heaviest....
and left space for these new elements, namely eka-silicon (germaniumGermaniumGermanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
), eka-aluminium (galliumGalliumGallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
), and eka-boron (scandiumScandiumScandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic transition metal, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids...
). Thus, there was no disturbance in the periodic table. - He pointed out that some of the then current atomic weights were incorrect.
- He provided for variance from atomic weight order.
Shortcomings of Mendeleev's table
- His table did not include any of the noble gasNoble gasThe noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es, which were discovered later. These were added by Sir William RamsayWilliam RamsaySir William Ramsay was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous elements in air" .-Early years:Ramsay was born in Glasgow on 2...
as Group 0, without any disturbance to the basic concept of the periodic table. - There was no place for the isotopeIsotopeIsotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of the various elements, which were discovered later.
Lothar Meyer
Unknown to Mendeleev, Lothar Meyer was also working on a periodic table. Although his work was published in 1864, and was done independently of Mendeleev, few historians regard him as an equal co-creator of the periodic table. For one thing, Meyer's table only included 28 elements. Furthermore, Meyer classified elements not by atomic weightAtomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
, but by valence
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
alone. Finally, Meyer never came to the idea of predicting new elements and correcting atomic weights. Only a few months after Mendeleev published his periodic table of all known elements (and predicted some new elements to complete the table, plus some corrected atomic weights), Meyer published a virtually identical table. While a few people consider Meyer and Mendeleev the co-creators of the periodic table, most agree that, by itself, Mendeleev's accurate prediction of the qualities of the undiscovered elements lands him the larger share of credit. In any case, at the time Mendeleev's predictions greatly impressed his contemporaries and were eventually found to be correct. An English chemist, William Odling
William Odling
William Odling, FRS was an English chemist who contributed to the development of the periodic table....
, also drew up a table that is remarkably similar to that of Mendeleev, in 1864.
Henry Moseley
In 1914 Henry MoseleyHenry Moseley
Henry Gwyn Jeffreys Moseley was an English physicist. Moseley's outstanding contribution to the science of physics was the justification from physical laws of the previous empirical and chemical concept of the atomic number. This stemmed from his development of Moseley's law in X-ray spectra...
found a relationship between an element's X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
wavelength and its atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
(Z), and therefore resequenced the table by nuclear charge rather than atomic weight. Before this discovery, atomic numbers were just sequential numbers based on an element's atomic weight. Moseley's discovery showed that atomic numbers had an experimentally measurable basis.
Thus Moseley placed argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
(Z=18) before potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
(Z=19) based on their X-ray wavelengths, despite the fact that argon has a greater atomic weight (39.9) than potassium (39.1). The new order agrees with the chemical properties of these elements, since argon is a noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
and potassium an alkali metal
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
. Similarly, Moseley placed cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
before nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, and was able to explain that tellurium occurs before iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
without revising the experimental atomic weight of tellurium (127.6) as proposed by Mendeleev.
Moseley's research also showed that there were gaps in his table at atomic numbers 43 and 61 which are now known to be Technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
and Promethium
Promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. It is notable for being the only exclusively radioactive element besides technetium that is followed by chemical elements with stable isotopes.- Prediction :...
, respectively, both radioactive and not naturally occurring. Following in the footsteps of Dmitri Mendeleev, Henry Moseley also predicted new elements.
Glenn T. Seaborg
During his Manhattan Project research in 1943 Glenn T. SeaborgGlenn T. Seaborg
Glenn Theodore Seaborg was an American scientist who won the 1951 Nobel Prize in Chemistry for "discoveries in the chemistry of the transuranium elements", contributed to the discovery and isolation of ten elements, and developed the actinide concept, which led to the current arrangement of the...
experienced unexpected difficulty isolating Americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
(95) and Curium
Curium
Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
(96). He began wondering if these elements more properly belonged to a different series which would explain why the expected chemical properties of the new elements were different. In 1945, he went against the advice of colleagues and proposed a significant change to Mendeleev's table: the actinide series.
Seaborg's actinide concept
Actinide concept
The actinide concept in nuclear chemistry was first theorized by Glenn T. Seaborg in 1944, resulting in the extension of Dmitri Mendeleev's periodic table of the elements by placing a new actinide series, for elements 89–103, below the lanthanide series...
of heavy element electronic structure, predicting that the actinides form a transition series analogous to the rare earth series of lanthanide elements, is now well accepted in the scientific community and included in all standard configurations of the periodic table. The actinide series are the second row of the f-block (5f series) and comprise the elements from Actinium to Lawrencium. Seaborg's subsequent elaborations of the actinide concept theorized a series of superheavy elements in a transactinide series comprising elements 104 through 121 and a superactinide series inclusive of elements 122 through 153.
Main discovery periods
The history of the periodic table is also a history of the discovery of the chemical elements.IUPAC suggest five "main discovery periods":
The periodic table as a cultural icon
Throughout the 20th century, the periodic table grew in ubiquity. Its presence on a classroom wall tells the movie-viewing audience that they are viewing a science classroom. It is often provided to students taking standardized tests as a necessary tool to complete chemical problems.In 1998, a 35-by-65 foot periodic table was constructed at the Science Museum of Virginia and is a Guinness World Record
Guinness World Records
Guinness World Records, known until 2000 as The Guinness Book of Records , is a reference book published annually, containing a collection of world records, both human achievements and the extremes of the natural world...
.
See also
- Prout's hypothesisProut's hypothesisProut's hypothesis was an early 19th century attempt to explain the existence of the various chemical elements through a hypothesis regarding the internal structure of the atom...
- History of chemistryHistory of chemistryBy 1000 BC, ancient civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, making pigments for cosmetics and painting, extracting chemicals from...
- Discoveries of the chemical elementsDiscoveries of the chemical elementsThe discovery of the elements known to exist today is presented here in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately defined.Given is each element's name,...
- Periodic tablePeriodic tableThe periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
- Periodic Systems of Small MoleculesPeriodic Systems of Small MoleculesPeriodic systems of molecules are charts of molecules similar to the periodic table of the elements. Construction of such charts was initiated in the early 20th century and is still ongoing....
- Alternative periodic tablesAlternative periodic tablesAlternative periodic tables are tabulations of chemical elements differing significantly in their organization from the traditional depiction of the Periodic System. Several have been devised, often purely for didactic reasons, as not all correlations between the chemical elements are effectively...
External links
- History of the Development of the Periodic Table of Elements
- Development of the periodic table
- Classification of the elements
- The path to the periodic table
- Web page listing several scholarly and semi-popular articles on various aspects of the periodic system and underlying theoretical concepts. Some are downloadable!
- Periodic Table Database

