
Phosphaalkyne
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, phosphaalkynes (IUPAC name: alkylidynephosphanes) are organophosphorus compounds that have a phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
.
Two types of phosphaalkynes are recognized. One type of phosphaalkyne is a heavier analogue of nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
s (R-C≡N), i.e. P replaces N in a nitrile. Another type of phosphaalkyne feature pentavalent, three coordinate phosphorus. Such species can also be described as ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
s or phosphinocarbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s. The phosphorus version of the isonitriles R-P+≡C-, which would be called isophosphaalkynes, has not been observed.
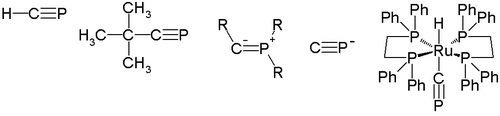
In 1950, H. Albers reported the first indication of the existence of the parent compound of phosphaalkynes (type A), H-C≡P
Methylidynephosphane
Methylidynephosphane is a chemical compound which was the first phosphaalkyne compound discovered, containing the unusual C≡P carbon-phosphorus triple bond. It is thus the phosphorus analogue of hydrogen cyanide, with the nitrile nitrogen replaced by phosphorus...
. This compound was identified by infrared absorption spectrometry, and its synthesis was improved by Manfred Regitz in 1987. The synthesis of the first kinetically stable phosphaalkyne, which has a tert-butyl group as a substituent R (tert-Butylphosphaacetylene
Tert-Butylphosphaacetylene
tert-Butylphosphaacetylene is an organophosphorus compound. Abbreviated t-BuC≡P, it was the first example of an isolable phosphaalkyne. Prior to its synthesis, the double bond rule had suggested that elements of Period 3 and higher were unable to form double or triple bonds with lighter main group...
), was reported in 1981 by Gerd Becker and Werner Uhl. These phosphaalkynes undergo 1,2-addition reaction
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
s and cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s .
In 2000, Guy Bertrand reported the first structure of the type B phosphaalkyne. Its P-C-R bond angle is 152.6 degrees, so this type of phosphaalkyne may be best described by a phosphorus vinyl ylide structure (B2).
Cyaphide ion
The cyaphide ion P≡C− as the phosphorus cyanideCyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
cousin is not known as a salt and only observed in the gas phase. In silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
measurements reveal that the -1 charge in this ion is location mainly on carbon (0.65). On the other hand the molecule does exist as a terminal ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in a certain ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
transition metal complex trans-[(dppe)2Ru(H)(C≡P)] stabilized by dppe.

