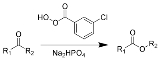
Baeyer-Villiger oxidation
Encyclopedia
The Baeyer–Villiger oxidation is an organic reaction
in which a ketone
is oxidized
to an ester
by treatment with peroxy acid
s or hydrogen peroxide
. Key features of the Baeyer–Villiger oxidation are its stereospecificity and predictable regiochemistry. It is named after the German chemist Johann Friedrich Wilhelm Adolf von Baeyer (1835–1917) and the Swiss chemist Victor Villiger
(1868–1934).
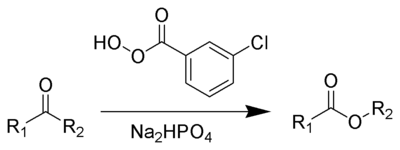 Reagent
Reagent
s typically used to carry out this rearrangement are meta-chloroperoxybenzoic acid
(mCPBA), peroxyacetic acid
, or peroxytrifluoroacetic acid. Reactive or strained ketones (cyclobutanones, norbornanones) react with hydrogen peroxide
or hydroperoxides to form lactones. The original reagent in the 1899 publication is Caro's acid discovered just a year earlier. Disodium phosphate
or sodium bicarbonate
is often added as a buffering agent
to prevent transesterification
or hydrolysis.
of this oxidative cleavage involves first addition of the peroxy acid to the carbonyl forming a tetrahedral
intermediate also called the Criegee intermediate for its similarity with rearrangement of that name. The transition state for this step is envisioned as a hydrogen relay involving three peroxy acid molecules with linear O-H-O interactions. Next is a concerted
migration of one of the adjacent carbons to oxygen with loss of a carboxylic acid
. If the migrating carbon is chiral, the stereochemistry is retained.
In the transition state
for this migration step the R-C-O-O dihedral angle
should be 180° in order to maximise the interaction between the filled R-C sigma bond
and the antibonding
O-O sigma bond. This step is also (at least in silico
) assisted by two or three peroxyacid units enabling the hydroxyl proton to shuttle to its new position.
For unsymmetrical ketones, the migrating group is usually the one that can best stabilize positive charge. Thus, cyclic ketones produce lactone
s and aldehydes usually produce carboxylic acid
s, although formate
s can also be formed if the migrating group is tertiary or an electron rich vinyl group or aromatic ring (Dakin reaction
). Sometimes the alcohol
is formed when the formate is hydrolytically unstable.
This mechanism was first proposed by Doering
and Dorfman in 1953 and based on isotope labeling experiments
with a so-called Baeyer-Villiger monooxygenase or BVMO. Though largely an experimental technique it shows the promise of enantioselectivity and green chemistry
for this reaction type. Current stumbling blocks are confinement to water as the reaction medium, substrate specificity, dependence on the stoichiometric
use and costs of cofactor
s such as NADPH and the costs associated with BVMO's themselves because lengthy purification steps are required. In vivo
oxidations with metabolically active microbial cells introduce complications on their own.
In one study the enzyme purification issue is addressed and a special thermally stable monooxygenase
is isolated from a specific E. coli
strain. This enzyme
converts racemic
2-phenylcyclohexanone with oxygen to the corresponding (R)-lactone
with 50% chemical yield and 94% enantiomeric excess
with in a biphasic system of water and hexane
. The NADPH cofactor is regenerated in each catalytic cycle
by action of a second dehydrogenase
enzyme which consumes isopropanol as a sacrificial catalyst.
The solubility of the organic reactant and product is low in the aqueous phase thus averting inhibition
. On the other hand the catalytic turnover number for this reaction is much larger than can be obtained with classical organic asymmetric catalysts.

Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
is oxidized
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
to an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
by treatment with peroxy acid
Peroxy acid
A peroxy acid is an acid which contains an acidic -OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the organic derivatives of carboxylic acids...
s or hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
. Key features of the Baeyer–Villiger oxidation are its stereospecificity and predictable regiochemistry. It is named after the German chemist Johann Friedrich Wilhelm Adolf von Baeyer (1835–1917) and the Swiss chemist Victor Villiger
Victor Villiger
Victor Villiger was a Swiss-born German chemist and the discoverer of the Baeyer-Villiger oxidation.-Life:...
(1868–1934).

Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s typically used to carry out this rearrangement are meta-chloroperoxybenzoic acid
Meta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling...
(mCPBA), peroxyacetic acid
Peroxyacetic acid
Peracetic acid , is an organic compound with the formula CH3CO3H. This organic peroxide is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid...
, or peroxytrifluoroacetic acid. Reactive or strained ketones (cyclobutanones, norbornanones) react with hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
or hydroperoxides to form lactones. The original reagent in the 1899 publication is Caro's acid discovered just a year earlier. Disodium phosphate
Disodium phosphate
Disodium hydrogen phosphate is a sodium salt of phosphoric acid. It is a white powder that is highly hygroscopic and water soluble. It is therefore used commercially as an anti-caking additive in powdered products. It is also known as disodium hydrogen orthophosphate, sodium hydrogen phosphate...
or sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
is often added as a buffering agent
Buffering agent
A buffering agent is a weak acid or base used to maintain the acidity of a solution at a chosen value. The function of a buffering agent is to prevent a rapid change in pH when acids or bases are added to the solution. Buffering agents have variable properties—some are more soluble than others;...
to prevent transesterification
Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R′ of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst...
or hydrolysis.
Mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of this oxidative cleavage involves first addition of the peroxy acid to the carbonyl forming a tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
intermediate also called the Criegee intermediate for its similarity with rearrangement of that name. The transition state for this step is envisioned as a hydrogen relay involving three peroxy acid molecules with linear O-H-O interactions. Next is a concerted
Concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not to depend on solvent polarity ruling out large buildup...
migration of one of the adjacent carbons to oxygen with loss of a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
. If the migrating carbon is chiral, the stereochemistry is retained.
- Migratory aptitude: H > tertiary alkyl > cyclohexyl > secondary alkyl, aryl > primary alkyl > methyl
In the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
for this migration step the R-C-O-O dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
should be 180° in order to maximise the interaction between the filled R-C sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
and the antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
O-O sigma bond. This step is also (at least in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
) assisted by two or three peroxyacid units enabling the hydroxyl proton to shuttle to its new position.
For unsymmetrical ketones, the migrating group is usually the one that can best stabilize positive charge. Thus, cyclic ketones produce lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s and aldehydes usually produce carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s, although formate
Formate
Formate or methanoate is the ion CHOO− or HCOO− . It is the simplest carboxylate anion. It is produced in large amounts in the hepatic mitochondria of embryonic cells and in cancer cells by the folate cycle Formate or methanoate is the ion CHOO− or HCOO− (formic acid minus one hydrogen ion). It...
s can also be formed if the migrating group is tertiary or an electron rich vinyl group or aromatic ring (Dakin reaction
Dakin reaction
The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate[3]...
). Sometimes the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
is formed when the formate is hydrolytically unstable.
This mechanism was first proposed by Doering
William von Eggers Doering
William von Eggers Doering was a Professor Emeritus at Harvard University and the former Chair of its Chemistry Department...
and Dorfman in 1953 and based on isotope labeling experiments
Biocatalytic BV oxidation
The Baeyer–Villiger oxidation can also be performed by biocatalysisBiocatalysis
Biocatalysis is the use of natural catalysts, such as protein enzymes, to perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task....
with a so-called Baeyer-Villiger monooxygenase or BVMO. Though largely an experimental technique it shows the promise of enantioselectivity and green chemistry
Green chemistry
Green chemistry, also called sustainable chemistry, is a philosophy of chemical research and engineering that encourages the design of products and processes that minimize the use and generation of hazardous substances...
for this reaction type. Current stumbling blocks are confinement to water as the reaction medium, substrate specificity, dependence on the stoichiometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
use and costs of cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
s such as NADPH and the costs associated with BVMO's themselves because lengthy purification steps are required. In vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
oxidations with metabolically active microbial cells introduce complications on their own.
In one study the enzyme purification issue is addressed and a special thermally stable monooxygenase
Oxygenase
An oxygenase is any enzyme that oxidizes a substrate by transferring the oxygen from molecular oxygen O2 to it. The oxygenases form a class of oxidoreductases; their EC number is EC 1.13 or EC 1.14....
is isolated from a specific E. coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
strain. This enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
converts racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
2-phenylcyclohexanone with oxygen to the corresponding (R)-lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
with 50% chemical yield and 94% enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
with in a biphasic system of water and hexane
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
. The NADPH cofactor is regenerated in each catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
by action of a second dehydrogenase
Dehydrogenase
A dehydrogenase is an enzyme that oxidises a substrate by a reduction reaction that transfers one or more hydrides to an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN.-Examples:...
enzyme which consumes isopropanol as a sacrificial catalyst.
The solubility of the organic reactant and product is low in the aqueous phase thus averting inhibition
Reaction inhibitor
A reaction inhibitor is a substance that decreases the rate of, or prevents, a chemical reaction.-Inhibition of a catalyst:An inhibitor can reduce the effectiveness of a catalyst in a catalysed reaction...
. On the other hand the catalytic turnover number for this reaction is much larger than can be obtained with classical organic asymmetric catalysts.


