Spirotryprostatin B
Encyclopedia
Spirotryprostatin B is an indolic
alkaloid
found in the Aspergillus fumigatus
fungus. Spirotryprostatin B and several other indolic alkaloids (including Spirotryprostatin A
, as well as other tryprostatins and cyclotryprostatins) have been found to have anti-mitotic
properties, and as such they have become of great interest as anti-cancer
drugs. Because of this, the total syntheses
of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
, with a number of other syntheses following shortly thereafter by Williams, Ganesan, Fuji, Carreira, Horne, Overman, and most recently Trost.
From a synthetic point of view, the most challenging structural features of the molecule are the C3 spirocyclic ring juncture and the adjacent prenyl-substituted carbon. Approaches toward preparing the skeleton of spirotryprostatin B have varied considerably.
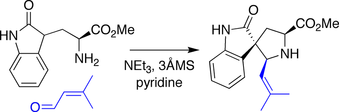
Danishefsky spirotryprostatin B synthesis
In the Danishefsky synthesis, an amine derived from tryptophan
was condensed with an aldehyde
, triggering a Mannich-type
reaction wherein the pendant oxindole
acted as a nucleophile
toward the intermediate iminium
species.
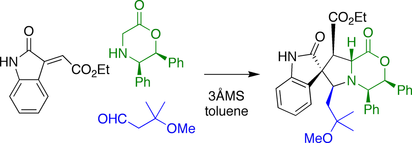
Williams spirotryprostatin B synthesis
The synthesis by the Williams group utilized a 3-component coupling reaction. A secondary amine
was combined with an aldehyde
to form an intermediate azomethine ylide
, which underwent a 1,3-dipolar cycloaddition
with an unsaturated oxindole
also present in the reaction mixture.
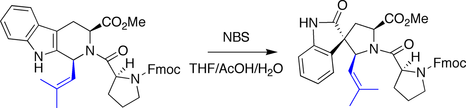
Ganesan spirotryprostatin B synthesis
Ganesan made use of a biomimetic strategy in his synthesis of spirotryprostatin B. An indole
was treated with N-bromosuccinimide
to trigger an oxidative rearrangement, forming the quaternary stereocenter
in a diastereoselective manner.
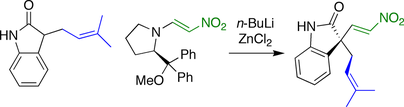
Fuji spirotryprostatin B synthesis
In the synthesis developed by the Fuji group, the stereochemistry
at the spirocyclic carbon was established by a nitroolefination reaction. An oxindole
with pendant prenyl group was reacted with a nitroolefin bearing a chiral
leaving group
.
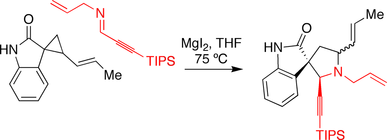
Carreira spirotryprostatin B synthesis
The Carreira group made use of a magnesium iodide promoted annulation reaction in their approach toward spirotryprostatin B. An oxindole
bearing a cyclopropane
was reacted with an imine
in the presence of the magnesium iodide, triggering the ring-expansion reaction.
Horne spirotryprostatin B synthesis
Horne's synthesis of spirotryprostatin B also made use of a Mannich-type
process, wherein a chloro-indole
served as the pro-nucleophile
. The cyclization was triggered by treating the pendant imine
with the acyl chloride
derived from proline
. The resulting iminium
species was attacked by the chloro-indole
, forming the spirocyclic bond.
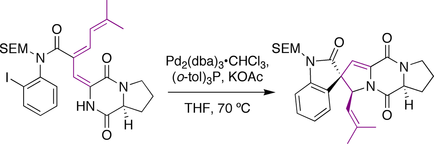
Overman spirotryprostatin B synthesis
The Overman group utilized a Heck reaction
to prepare the molecule. An iodo-aniline
with a tethered alkene
was subjected to palladium
catalysis
. The intermediate palladium-allyl species was intercepted by the pendant amide
nitrogen to generate the prenyl stereocenter
in the same reaction.
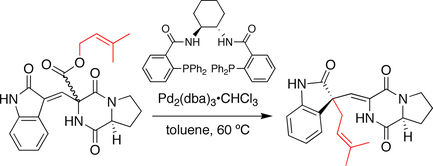
Trost spirotryprostatin B synthesis
In the synthesis developed by the Trost group, the stereochemistry at the spirocyclic ring juncture is established by a decarboxylation-prenylation sequence, reminiscent of the Carroll reaction. Here, a prenyl ester serves as both the nucleophile
and electrophile
precursor. Upon treatment with a chiral
palladium catalyst the prenyl group ionizes and decarboxylates. The resulting ion
pair subsequently recombines to generate the prenylated product. Notably, double bond migration occurs and the prenyl group is attacked at the oxindole
carbon.
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
alkaloid
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
found in the Aspergillus fumigatus
Aspergillus fumigatus
Aspergillus fumigatus is a fungus of the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency....
fungus. Spirotryprostatin B and several other indolic alkaloids (including Spirotryprostatin A
Spirotryprostatin A
Spirotryprostatin A is an indolic alkaloid found in the Aspergillus fumigatus fungus. Spirotryprostatin A and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs...
, as well as other tryprostatins and cyclotryprostatins) have been found to have anti-mitotic
Mitosis
Mitosis is the process by which a eukaryotic cell separates the chromosomes in its cell nucleus into two identical sets, in two separate nuclei. It is generally followed immediately by cytokinesis, which divides the nuclei, cytoplasm, organelles and cell membrane into two cells containing roughly...
properties, and as such they have become of great interest as anti-cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
drugs. Because of this, the total syntheses
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
Total synthesis
The first total synthesis was accomplished in 2000 by the Danishefsky group at Columbia UniversityColumbia University
Columbia University in the City of New York is a private, Ivy League university in Manhattan, New York City. Columbia is the oldest institution of higher learning in the state of New York, the fifth oldest in the United States, and one of the country's nine Colonial Colleges founded before the...
, with a number of other syntheses following shortly thereafter by Williams, Ganesan, Fuji, Carreira, Horne, Overman, and most recently Trost.
From a synthetic point of view, the most challenging structural features of the molecule are the C3 spirocyclic ring juncture and the adjacent prenyl-substituted carbon. Approaches toward preparing the skeleton of spirotryprostatin B have varied considerably.
Danishefsky spirotryprostatin B synthesis
In the Danishefsky synthesis, an amine derived from tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
was condensed with an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
, triggering a Mannich-type
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
reaction wherein the pendant oxindole
Oxindole
Oxindole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring...
acted as a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
toward the intermediate iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
species.
Williams spirotryprostatin B synthesis
The synthesis by the Williams group utilized a 3-component coupling reaction. A secondary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
was combined with an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
to form an intermediate azomethine ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
, which underwent a 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
with an unsaturated oxindole
Oxindole
Oxindole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring...
also present in the reaction mixture.
Ganesan spirotryprostatin B synthesis
Ganesan made use of a biomimetic strategy in his synthesis of spirotryprostatin B. An indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
was treated with N-bromosuccinimide
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
to trigger an oxidative rearrangement, forming the quaternary stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
in a diastereoselective manner.
Fuji spirotryprostatin B synthesis
In the synthesis developed by the Fuji group, the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
at the spirocyclic carbon was established by a nitroolefination reaction. An oxindole
Oxindole
Oxindole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring...
with pendant prenyl group was reacted with a nitroolefin bearing a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
.
Carreira spirotryprostatin B synthesis
The Carreira group made use of a magnesium iodide promoted annulation reaction in their approach toward spirotryprostatin B. An oxindole
Oxindole
Oxindole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring...
bearing a cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
was reacted with an imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
in the presence of the magnesium iodide, triggering the ring-expansion reaction.
Horne spirotryprostatin B synthesis
Horne's synthesis of spirotryprostatin B also made use of a Mannich-type
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
process, wherein a chloro-indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
served as the pro-nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. The cyclization was triggered by treating the pendant imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
with the acyl chloride
Acyl chloride
In organic chemistry, an acyl chloride is an organic compound with the functional group -CO-Cl. Their formula is usually written RCOCl, where R is a side chain. They are usually considered to be reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride,...
derived from proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
. The resulting iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
species was attacked by the chloro-indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
, forming the spirocyclic bond.
Overman spirotryprostatin B synthesis
The Overman group utilized a Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
to prepare the molecule. An iodo-aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
with a tethered alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
was subjected to palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. The intermediate palladium-allyl species was intercepted by the pendant amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
nitrogen to generate the prenyl stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
in the same reaction.
Trost spirotryprostatin B synthesis
In the synthesis developed by the Trost group, the stereochemistry at the spirocyclic ring juncture is established by a decarboxylation-prenylation sequence, reminiscent of the Carroll reaction. Here, a prenyl ester serves as both the nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
and electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
precursor. Upon treatment with a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
palladium catalyst the prenyl group ionizes and decarboxylates. The resulting ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
pair subsequently recombines to generate the prenylated product. Notably, double bond migration occurs and the prenyl group is attacked at the oxindole
Oxindole
Oxindole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring...
carbon.