
Phosphogluconate dehydrogenase (decarboxylating)
Encyclopedia
In enzymology, a phosphogluconate dehydrogenase (decarboxylating) is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are 6-phospho-D-gluconate and NADP+
, whereas its 3 products
are D-ribulose 5-phosphate, CO2
, and NADPH
.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is 6-phospho-D-gluconate:NADP+ 2-oxidoreductase (decarboxylating). Other names in common use include phosphogluconic acid dehydrogenase, 6-phosphogluconic dehydrogenase, 6-phosphogluconic carboxylase, 6-phosphogluconate dehydrogenase (decarboxylating), and 6-phospho-D-gluconate dehydrogenase. This enzyme participates in pentose phosphate pathway
. It employs one cofactor
, manganese
.
, with each subunit containing three domains. The N-terminal coenzyme binding domain contains a Rossmann fold
with additional α/β units. The second domain consists of a number of alpha helical structures, and the C-terminal domain consists of a short tail. The tails of the two subunits interact with each other to form a mobile lid on the enzyme’s active site.
As of late 2007, 11 structures
have been solved for this class of enzymes, with PDB
accession codes , , , , , , , , , , and .
, carbon dioxide
, and NADPH is believed to follow a sequential mechanism with ordered product release. 6-phosphogluconate is first oxidized to 3-keto-6-phosphogluconate and NADPH is formed and released. Then, the intermediate is decarboxylated, yielding a 1,2-enediol of ribulose 5-phosphate, which tautomerizes to form ribulose 5-phosphate. High levels of NADPH are believed to inhibit the enzyme, while 6-phosphogluconate acts to activate the enzyme.
synthesis, and functions in the pentose phosphate pathway
as the main generator of cellular NADPH.
and glutathione reductase
to reduce oxidized thioredoxin
and glutathionine, 6-phosphogluconate dehydrogenase is believed to be involved in protecting cells from oxidative damage. Several studies have linked oxidative stress to diseases such as Alzheimer’s disease, as well as cancer
, These studies have found phosphogluconate dehydrogenase activity to be up-regulated, both in tumor cells and in relevant cortical regions of Alzheimer’s patient brains, most likely as a compensatory reaction to highly oxidative environments.
Recently, phosphogluconate dehydrogenase has been posited as a potential drug target for African sleeping sickness (trypanosomiasis
). The pentose phosphate pathway protects the trypanosomes from oxidative stress via the generation of NADPH and provides carbohydrate intermediates used in nucleotide synthesis. Structural differences between mammalian and trypanosome 6-phosphogluconate dehydrogenase have allowed for the development of selective inhibitors of the enzyme. Phosphorylated carbohydrate substrate and transition state analogues, non-carbohydrate substrate analogues and triphenylmethane-based compounds are currently being explored.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- 6-phospho-D-gluconate + NADP+
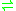 D-ribulose 5-phosphate + CO2 + NADPH
D-ribulose 5-phosphate + CO2 + NADPH
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are 6-phospho-D-gluconate and NADP+
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
, whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are D-ribulose 5-phosphate, CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, and NADPH
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is 6-phospho-D-gluconate:NADP+ 2-oxidoreductase (decarboxylating). Other names in common use include phosphogluconic acid dehydrogenase, 6-phosphogluconic dehydrogenase, 6-phosphogluconic carboxylase, 6-phosphogluconate dehydrogenase (decarboxylating), and 6-phospho-D-gluconate dehydrogenase. This enzyme participates in pentose phosphate pathway
Pentose phosphate pathway
The pentose phosphate pathway is a process that generates NADPH and pentoses . There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars...
. It employs one cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
, manganese
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
.
Enzyme Structure
The general structure, as well as several critical residues, on 6-phosphogluconate dehydrogenase appear to be well conserved over various species. The enzyme is a dimerProtein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
, with each subunit containing three domains. The N-terminal coenzyme binding domain contains a Rossmann fold
Rossmann fold
The Rossmann fold is a protein structural motif found in proteins that bind nucleotides, especially the cofactor NAD. The structure with two repeats is composed of six parallel beta strands linked to two pairs of alpha helices in the topological order beta-alpha-beta-alpha-beta...
with additional α/β units. The second domain consists of a number of alpha helical structures, and the C-terminal domain consists of a short tail. The tails of the two subunits interact with each other to form a mobile lid on the enzyme’s active site.
As of late 2007, 11 structures
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , , , , , , and .
Enzyme Mechanism
The conversion of 6-phosphogluconate and NADP to ribulose 5-phosphateRibulose 5-phosphate
Ribulose 5-phosphate is one of the end-products of the pentose phosphate pathway. It is also an intermediate in the Calvin cycle.It is formed by phosphogluconate dehydrogenase, and it can be acted upon by phosphopentose isomerase and phosphopentose epimerase....
, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, and NADPH is believed to follow a sequential mechanism with ordered product release. 6-phosphogluconate is first oxidized to 3-keto-6-phosphogluconate and NADPH is formed and released. Then, the intermediate is decarboxylated, yielding a 1,2-enediol of ribulose 5-phosphate, which tautomerizes to form ribulose 5-phosphate. High levels of NADPH are believed to inhibit the enzyme, while 6-phosphogluconate acts to activate the enzyme.
Biological Function
6-phosphogluconate dehydrogenase is involved in the production of ribulose 5-phosphate, which is used in nucleotideNucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
synthesis, and functions in the pentose phosphate pathway
Pentose phosphate pathway
The pentose phosphate pathway is a process that generates NADPH and pentoses . There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars...
as the main generator of cellular NADPH.
Disease Relevance
Since NADPH is required by both thioredoxin reductaseThioredoxin reductase
Thioredoxin Reductases are the only known enzymes to reduce thioredoxin . Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Both classes are flavoproteins which function as homodimers...
and glutathione reductase
Glutathione reductase
Glutathione reductase, also known as GSR or GR, is an enzyme that reduces glutathione disulfide to the sulfhydryl form GSH, which is an important cellular antioxidant....
to reduce oxidized thioredoxin
Thioredoxin
Thioredoxin is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes. In humans, it is encoded by the TXN gene. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the...
and glutathionine, 6-phosphogluconate dehydrogenase is believed to be involved in protecting cells from oxidative damage. Several studies have linked oxidative stress to diseases such as Alzheimer’s disease, as well as cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
, These studies have found phosphogluconate dehydrogenase activity to be up-regulated, both in tumor cells and in relevant cortical regions of Alzheimer’s patient brains, most likely as a compensatory reaction to highly oxidative environments.
Recently, phosphogluconate dehydrogenase has been posited as a potential drug target for African sleeping sickness (trypanosomiasis
Trypanosomiasis
Trypanosomiasis or trypanosomosis is the name of several diseases in vertebrates caused by parasitic protozoan trypanosomes of the genus Trypanosoma. Approximately 500,000 men, women and children in 36 countries of sub-Saharan Africa suffer from human African trypanosomiasis which is caused by...
). The pentose phosphate pathway protects the trypanosomes from oxidative stress via the generation of NADPH and provides carbohydrate intermediates used in nucleotide synthesis. Structural differences between mammalian and trypanosome 6-phosphogluconate dehydrogenase have allowed for the development of selective inhibitors of the enzyme. Phosphorylated carbohydrate substrate and transition state analogues, non-carbohydrate substrate analogues and triphenylmethane-based compounds are currently being explored.

