Thioredoxin reductase
Encyclopedia
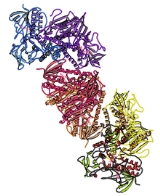
Thioredoxin Reductases (TR, TrxR) are the only known enzymes to reduce thioredoxin
(Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Both classes are flavoproteins which function as homodimers. Each monomer contains a FAD
prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond
.
These two classes of TrxR have only ~20% sequence identity in the section of primary sequence where they can be reliably aligned. The net reaction of both classes of TrxR is identical but the mechanism of action of each is distinct.
Humans express three thioredoxin reductase isozymes: TrxR1 (cytosolic), TrxR2 (mitochondrial), TrxR3 (testis specific). Each isozyme is encoded by a separate gene:
and another for NADPH. The connection between these two domains is a two-stranded anti-parallel f3-sheet. Each domain individually is very similar to the analogous domains in glutathione reductase
, and lipoamide dehydrogenase but they relative orientation of these domains in ThxR is rotated by 66 degrees. This becomes significant in the enzyme mechanism of action which is described below.
ThxR homo-dimerizes with the interface between the two monomers formed by three alpha-helicies and two loops. Each monomer can separately bind a molecule of thioredoxin
.
Mammalian thioredoxin reductase structure: Mammalian TrxR structure is similar to E. coli. It contains a FAD
and NADPH binding domain, and an interface between two monomer subunits. In mammalian ThxR there is an insertion in the FAD
binding domain between two alpha helices which forms a small pair of beta strands. The active disulfide in the enzyme is located on one of these helices and thus the active disulfide bond is located in the FAD
domain and not the NADPH domain as in E. coli and other prokaryotes.
Mammalian thioredoxin reductase mechanism: Mammalian TrxRs have a much higher sequence homology with glutathione reductase than E. coli. The active-site Cys residues in the FAD domain and bound NADPH domain are in close proximity removing the necessity for a 66 degree rotation for electron transfer found in E. coli. An additional feature of the mammalian mechanism is the presence of a selenocystine residue at the C-terminal end of the protein which is required for catalytic activity. The conserved residues in mammalian active site are -Cys-Val-Asn-Val-Gly-Cys-.
(MGd) is a new chemotherapeutic agent that selectively targets tumor cells, leading to cell death and apoptosis via inhibition of thioredoxin reductase and ribonucleotide reductase
.
- In cardiomyopathy: Dilated cardiomyopathy (DCM
) is a common diagnoses in cases of congestive heart failure
. Thioredoxin reductases are essential proteins for regulating cellular redox balance and mitigating the damage caused by reactive oxygen species
generated via oxidative phosphorylation
in the mitochondria. Inactivation of mitochondrial TrxR2 in mice results in thinning of the ventricular heart walls and embryonic death. Furthermore two mutations in the TrxR2 gene are found in patients diagnosed with DCM and not in a control population. It is hypothesized that the pathological impact of these mutations is an impaired ability to control oxidative damage in cardiac myocytes
.
Thioredoxin
Thioredoxin is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes. In humans, it is encoded by the TXN gene. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the...
(Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Both classes are flavoproteins which function as homodimers. Each monomer contains a FAD
FAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
.
Cellular Role
Thioredoxin reductase is the only enzyme known to catalyze the reduction of thioredoxin and hence is a central component in the thioredoxin system. Together with thioredoxin (Thx) and NADPH this system's most general description is as a method of forming reduced disulfide bonds in cells. Electrons are taken from NADPH via TrxR and are transferred to the active site of Trx, which goes on to reduce protein disulfides or other substrates. The Trx system exists in all living cells and has an evolutionary history tied to DNA as a genetic material, defense against oxidative damage due to oxygen metabolism, and redox signaling using molecules like hydrogen peroxide and nitric oxide.Diversity
Two classes of thioredoxin reductase have evolved independently:- A high molecular weight (MW = ~55,000) type containing a selenocysteineSelenocysteineSelenocysteine is an amino acid that is present in several enzymes .-Nomenclature:...
residue in its active site has been identified in higher eukaryotes including humans. This TxR is related to glutathione reductaseGlutathione reductaseGlutathione reductase, also known as GSR or GR, is an enzyme that reduces glutathione disulfide to the sulfhydryl form GSH, which is an important cellular antioxidant....
, trypanothione reductaseTrypanothione-disulfide reductaseIn enzymology, a trypanothione-disulfide reductase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are trypanothione and NADP+, whereas its 3 products are trypanothione disulfide, NADPH, and H+....
, mercuric reductase and lipoamide dehydrogenaseDihydrolipoamide dehydrogenaseDihydrolipoamide dehydrogenase , also known as dihydrolipoyl dehydrogenase, mitochondrial, is an enzyme that in humans is encoded by the DLD gene. DLD is a flavoprotein enzyme that degrades lipoamide, and produces dihydrolipoamide....
. - A low molecular weight (MW = ~ 35,000) type has been identified in archaea, bacteria and other eukarya.
These two classes of TrxR have only ~20% sequence identity in the section of primary sequence where they can be reliably aligned. The net reaction of both classes of TrxR is identical but the mechanism of action of each is distinct.
Humans express three thioredoxin reductase isozymes: TrxR1 (cytosolic), TrxR2 (mitochondrial), TrxR3 (testis specific). Each isozyme is encoded by a separate gene:
Structure
E. coli thioredoxin reductase structure: In E. coli ThxR there is are two binding domains, one for FADFAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
and another for NADPH. The connection between these two domains is a two-stranded anti-parallel f3-sheet. Each domain individually is very similar to the analogous domains in glutathione reductase
Glutathione reductase
Glutathione reductase, also known as GSR or GR, is an enzyme that reduces glutathione disulfide to the sulfhydryl form GSH, which is an important cellular antioxidant....
, and lipoamide dehydrogenase but they relative orientation of these domains in ThxR is rotated by 66 degrees. This becomes significant in the enzyme mechanism of action which is described below.
ThxR homo-dimerizes with the interface between the two monomers formed by three alpha-helicies and two loops. Each monomer can separately bind a molecule of thioredoxin
Thioredoxin
Thioredoxin is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes. In humans, it is encoded by the TXN gene. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the...
.
Mammalian thioredoxin reductase structure: Mammalian TrxR structure is similar to E. coli. It contains a FAD
FAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
and NADPH binding domain, and an interface between two monomer subunits. In mammalian ThxR there is an insertion in the FAD
FAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
binding domain between two alpha helices which forms a small pair of beta strands. The active disulfide in the enzyme is located on one of these helices and thus the active disulfide bond is located in the FAD
FAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
domain and not the NADPH domain as in E. coli and other prokaryotes.
Mechanism
E. coli thioredoxin reductase mechanism: In E. coli ThxR the spatial orientation of the FAD and NADPH domains are such that the redox-active rings of FAD and NADPH are not in close proximity to each other. When the FAD domain of E. coli is rotated 66 degrees with the NADPH domain remaining fixed the two prosthetic groups move into close contact allowing electrons to pass from NADPH to FAD and then to the active site disulfide bond. The conserved active site residues in E. coli are -Cys-Ala-Thr-Cys-.Mammalian thioredoxin reductase mechanism: Mammalian TrxRs have a much higher sequence homology with glutathione reductase than E. coli. The active-site Cys residues in the FAD domain and bound NADPH domain are in close proximity removing the necessity for a 66 degree rotation for electron transfer found in E. coli. An additional feature of the mammalian mechanism is the presence of a selenocystine residue at the C-terminal end of the protein which is required for catalytic activity. The conserved residues in mammalian active site are -Cys-Val-Asn-Val-Gly-Cys-.
Clinical significance
- In cancer treatment: Since the activity of this enzyme is essential for cell growth and survival, it is a good target for anti-tumor therapy. Furthermore, the enzyme is upregulated in several types of cancer, including malignant mesothelioma. For example, motexafin gadoliniumMotexafin gadolinium
Motexafin gadolinium is an inhibitor of thioredoxin reductase and ribonucleotide reductase. It has been proposed as a possible chemotherapeutic agent in the treatment of brain cancer.-History:...
(MGd) is a new chemotherapeutic agent that selectively targets tumor cells, leading to cell death and apoptosis via inhibition of thioredoxin reductase and ribonucleotide reductase
Ribonucleotide reductase
Ribonucleotide reductase is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms...
.
- In cardiomyopathy: Dilated cardiomyopathy (DCM
Dilated cardiomyopathy
Dilated cardiomyopathy or DCM is a condition in which the heart becomes weakened and enlarged and cannot pump blood efficiently. The decreased heart function can affect the lungs, liver, and other body systems....
) is a common diagnoses in cases of congestive heart failure
Congestive heart failure
Heart failure often called congestive heart failure is generally defined as the inability of the heart to supply sufficient blood flow to meet the needs of the body. Heart failure can cause a number of symptoms including shortness of breath, leg swelling, and exercise intolerance. The condition...
. Thioredoxin reductases are essential proteins for regulating cellular redox balance and mitigating the damage caused by reactive oxygen species
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
generated via oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
in the mitochondria. Inactivation of mitochondrial TrxR2 in mice results in thinning of the ventricular heart walls and embryonic death. Furthermore two mutations in the TrxR2 gene are found in patients diagnosed with DCM and not in a control population. It is hypothesized that the pathological impact of these mutations is an impaired ability to control oxidative damage in cardiac myocytes
Cardiac muscle
Cardiac muscle is a type of involuntary striated muscle found in the walls and histologic foundation of the heart, specifically the myocardium. Cardiac muscle is one of three major types of muscle, the others being skeletal and smooth muscle...
.

