
Cystathionine beta synthase
Encyclopedia
Cystathionine-β-synthase, also known as CBS, is an enzyme
that in humans is encoded by the CBS gene
. It catalyzes the first step of the transsulfuration pathway, from homocysteine
to cystathionine
:
CBS uses the cofactor
pyridoxal-phosphate
(PLP) and can be allosterically regulated by effectors such as the ubiquitous cofactor S-adenosyl-L-methionine (adoMet). This enzyme belongs to the family of lyase
s, to be specific, the hydro-lyases, which cleave carbon-oxygen bonds.
CBS is a multidomain enzyme composed of an N-terminal enzymatic domain and two CBS domain
s. The CBS gene is the most common locus for mutations associated with homocystinuria
.
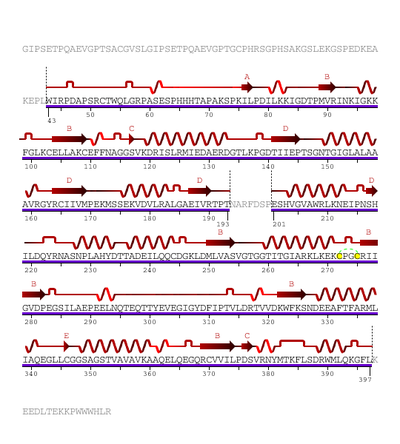 The human enzyme cystathionine β-synthase is a tetramer and comprises 551 amino acid
The human enzyme cystathionine β-synthase is a tetramer and comprises 551 amino acid
s with a subunit molecular weight of 63 kDa. It displays a modular organization of three modules with the N-terminal heme domain followed by a core that contains the PLP
cofactor. The cofactor is deep in the heme domain and is linked by a Schiff base. A Schiff base
is a functional group
containing a C=N bond with the nitrogen atom connected to an aryl
or alkyl group. The heme domain is composed of 70 amino acids and it appears that the heme only exists in mammal
ian CBS and is absent in yeast and protozoan CBS. At the C-terminus, the regulatory domain of CBS contains a tandem repeat of two CBS domains of β-α-β-β-α, a secondary structure motif found in other proteins. CBS has a C-terminal inhibitory domain. The C-terminal domain of cystathionine β-synthase regulates its activity via both intrasteric and allosteric effects and is important for maintaining the tetrameric state of the protein. This inhibition is alleviated by binding of the allosteric effector, adoMet
, or by deletion of the regulatory domain; however, the magnitude of the effects differ. Mutations in this domain are correlated with hereditary diseases.
The heme domain contains an N-terminal loop that binds heme and provides the axial ligand
s C52 and H65. The distance of heme from the PLP
binding site suggests its non-role in catalysis, however deletion of the heme domain causes loss of redox
sensitivity, therefore it is hypothesized that heme is a redox sensor. The presence of protoporphyrin IX in CBS is a unique PLP-dependent enzyme and is only found in the mammalian CBS. D. melanogaster
and D. discoides have truncated N-terminal extensions and therefore prevent the conserved histidine
and cysteine
heme ligand residue
s. However, the Anopheles gambiae
sequence has a longer N-terminal extension than the human enzyme and contains the conserved histidine
and cysteine
heme
ligand residues like the human heme
. Therefore, it is possible that CBS in slime molds and insects are hemeproteins that suggest that the heme
domain is an early evolutionary innovation that arose before the separation of animals and the slime molds. The PLP
is an internal aldimine
and forms a Schiff base
with K119 in the active site. Between the catalytic and regulatory domains exists a hypersensitive site that causes proteolytic cleavage and produces a truncated dimer
ic enzyme that is more active than the original enzyme. Both truncated enzyme and the enzyme found in yeast are not regulated by adoMet. The yeast enzyme is also activated by the deletion of the C-terminal to produce the dimeric enzyme.
As of late 2007, two structures
have been solved for this class of enzymes, with PDB
accession codes and .
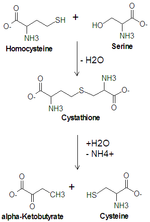 Transsulfuration, catalyzed by CBS, converts homocysteine
Transsulfuration, catalyzed by CBS, converts homocysteine
to cystathionine
, which cystathione gamma lyase converts to cysteine
.
CBS occupies a pivotal position in mammalian sulfur metabolism at the homocysteine
junction where the decision to conserve methionine
or to convert it to cysteine via the transsulfuration pathway
, is made. Moreover, the transsulfuration pathway is the only pathway capable of removing sulfur-containing amino acids under conditions of excess.
In analogy with other β-replacement enzymes, the reaction catalyzed by CBS is predicted to involve a series of adoMet-bound intermediates. Addition of serine
results in a transchiffization
reaction, which forms of an external aldimine
. The aldimine
undergoes proton abstraction at the α-carbon followed by elimination to generate an amino-acrylate
intermediate. Nucleophilic attack by the thiolate of homocysteine on the aminoacrylate and reprotonation at Cα generate the external aldimine of cystathionine
. A final transaldimination reaction releases the final product, cystathionine. The final product, L-cystathionine can also form an aminoacrylate intermediate, indicating that the entire reaction of CBS is reversible.
The measured V0 of an enzyme-catalyzed reaction, in general, reflects the steady state (where [ES] is constant), even though V0 is limited to the early part of a reaction, and analysis of these initial rates is referred to as steady-state kinetics. Steady-state kinetic analysis of yeast CBS yields parallel lines. These results agree with the proposed ping-pong mechanism in which serine binding and release of water are followed by homocysteine binding and release of cystathionine. In contrast, the steady-state enzyme kinetics
of rat CBS yields intersecting lines, indicating that the β-substitutent of serine is not released from the enzyme prior to binding of homocysteine.
One of the alternate reactions involving CBS is the condensation of cysteine
with homocysteine to form cystathionine and hydrogen sulfide
(H2S). H2S in the brain is produced from L-cysteine by CBS. This alternative metabolic pathway is also dependent on adoMet.
CBS enzyme activity is not found in all tissues and cells. It is absent from heart, lung, testes, adrenal, and spleen in rats. In humans, it has been shown to be absent in heart muscle and primary cultures of human aortic endothelial cells. The lack of CBS in these tissues implies that these tissues are unable to synthesize cysteine and that cysteine must be supplied from extracellular sources. It also suggests that these tissues might have increased sensitivity to homocysteine toxicity because they cannot catabolize excess homocysteine via transsulfuration.
Human CBS performs a crucial step in the biosynthetic pathway of cysteine by providing a regulatory control point for adoMet . L-homocysteine, after being methylated to methionine
, can be converted to adoMet , which donates methyl groups to a variety of substrates, e.g., neurotransmitters, proteins, and nucleic acids. adoMet functions as an allosteric activator of CBS and exerts control on its biosynthesis: low concentrations of adoMet result in low CBS activity, thereby funneling homocysteine in the transmethylation pathway toward adoMet formation. Methionine is transmethylated to homocysteine via S-adenosyl-methionine (SAM). In contrast, high adoMet concentrations allow the clearance of homocysteine into the transsulfuration pathway, leading to cysteine biosynthesis.
In mammals, CBS is a highly regulated enzyme, which contains a heme
cofactor that functions as a redox sensor, that can modulate its activity in response to changes in the redox potential. If the resting form of CBS in the cell has ferrous heme, the potential exists for activating the enzyme under oxidizing conditions by conversion to the ferric state. The Fe (II) form of the enzyme is inhibited upon binding CO or nitric oxide, whereas enzyme activity is doubled when the Fe (II) is oxidized to Fe (III). The redox state of the heme
is pH dependent, with oxidation of Fe (II)-CBS to Fe (III)-CBS being favored at low pH conditions.
Since mammalian CBS contains a heme cofactor, whereas yeast and protozoan enzyme from Trypanosoma cruzi do not have heme cofactors, researchers have speculated that heme is not required for CBS activity.
CBS is regulated at the transcriptional level by NF-Y, SP-1, and SP-3. In addition it is upregulated transcriptionally by glucocorticoids and glucogen, and downregulated by insulin. Methionine upregulates CBS at the post-transcriptional level.
is a medical condition characterized by an overexpression of cystathionine beta synthase (CBS) and a low level of homocysteine in the blood.
It has been speculated that cystathionine beta synthase overexpression could be the major culprit in this disease (along with dysfunctioning of GabaA and Dyrk1a). The phenotype of down syndrome is the opposite of Hyperhomocysteinemia (described below). Pharmacologicals inhibitors of CBS have been patented by the Jerome Lejeune Foundation (november 2011) and trials (animals and humans are planned).
Hyperhomocysteinemia
is a medical condition characterized by an abnormally large level of homocysteine
in the blood. Mutations in CBS are the single most common cause of hereditary hyperhomocysteinemia. Inborn errors in CBS result in hyperhomocysteinemia
with complications in the cardiovascular system leading to early and aggressive arterial disease. Hyperhomocysteinemia
also affects three other major organ systems including the ocular, central nervous, and skeletal.
Homocystinuria
due to CBS deficiency is a special type of hyperhomocysteinemia. It is a rare, hereditary recessive autosomal disease, in general, diagnosed during childhood. A total of 131 different homocystinuria-causing mutations have been identified. A common functional feature of the mutations in the CBS domains is that the mutations abolish or strongly reduce activation by adoMet. No specific cure has been discovered for homocystinuria; however, many people are treated using high doses of vitamin B6
, which is a cofactor of CBS.
development. However, little is known about the regional and cellular expression patterns of CBS in the ovary and research is now focused on determining the location and expression during follicle development in the ovaries.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that in humans is encoded by the CBS gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
. It catalyzes the first step of the transsulfuration pathway, from homocysteine
Homocysteine
Homocysteine is a non-protein amino acid with the formula HSCH2CH2CHCO2H. It is a homologue of the amino acid cysteine, differing by an additional methylene group. It is biosynthesized from methionine by the removal of its terminal Cε methyl group...
to cystathionine
Cystathionine
Cystathionine is an intermediate in the synthesis of cysteine.It is generated from homocysteine and serine by cystathionine beta synthase.It is cleaved into cysteine and α-ketobutyrate by cystathionine gamma-lyase....
:
- L-serineSerineSerine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
+ L-homocysteineHomocysteineHomocysteine is a non-protein amino acid with the formula HSCH2CH2CHCO2H. It is a homologue of the amino acid cysteine, differing by an additional methylene group. It is biosynthesized from methionine by the removal of its terminal Cε methyl group...
 L-cystathionineCystathionineCystathionine is an intermediate in the synthesis of cysteine.It is generated from homocysteine and serine by cystathionine beta synthase.It is cleaved into cysteine and α-ketobutyrate by cystathionine gamma-lyase....
L-cystathionineCystathionineCystathionine is an intermediate in the synthesis of cysteine.It is generated from homocysteine and serine by cystathionine beta synthase.It is cleaved into cysteine and α-ketobutyrate by cystathionine gamma-lyase....
+ H2O
CBS uses the cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
pyridoxal-phosphate
Pyridoxal-phosphate
Pyridoxal-phosphate is a prosthetic group of some enzymes. It is the active form of vitamin B6, which comprises three natural organic compounds, pyridoxal, pyridoxamine and pyridoxine.-Role as a coenzyme:...
(PLP) and can be allosterically regulated by effectors such as the ubiquitous cofactor S-adenosyl-L-methionine (adoMet). This enzyme belongs to the family of lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, to be specific, the hydro-lyases, which cleave carbon-oxygen bonds.
CBS is a multidomain enzyme composed of an N-terminal enzymatic domain and two CBS domain
CBS domain
The CBS domain is a protein domain found in a range of proteins in all species from bacteria to man. It was first identified as a conserved sequence region in 1997 and named after cystathionine beta synthase, one of the proteins it is found in...
s. The CBS gene is the most common locus for mutations associated with homocystinuria
Homocystinuria
Homocystinuria, also known as cystathionine beta synthase deficiency or CBS deficiency, is an inherited disorder of the metabolism of the amino acid methionine, often involving cystathionine beta synthase...
.
Nomenclature
The systematic name of this enzyme class is L-serine hydro-lyase (adding homocysteine; L-cystathionine-forming). Other names in common use include:- beta-thionase,
- cysteine synthase,
- L-serine hydro-lyase (adding homocysteine),
- methylcysteine synthase,
- serine sulfhydrase, and
- serine sulfhydrylase.
Structure

Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s with a subunit molecular weight of 63 kDa. It displays a modular organization of three modules with the N-terminal heme domain followed by a core that contains the PLP
Pyridoxal-phosphate
Pyridoxal-phosphate is a prosthetic group of some enzymes. It is the active form of vitamin B6, which comprises three natural organic compounds, pyridoxal, pyridoxamine and pyridoxine.-Role as a coenzyme:...
cofactor. The cofactor is deep in the heme domain and is linked by a Schiff base. A Schiff base
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
is a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
containing a C=N bond with the nitrogen atom connected to an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
or alkyl group. The heme domain is composed of 70 amino acids and it appears that the heme only exists in mammal
Mammal
Mammals are members of a class of air-breathing vertebrate animals characterised by the possession of endothermy, hair, three middle ear bones, and mammary glands functional in mothers with young...
ian CBS and is absent in yeast and protozoan CBS. At the C-terminus, the regulatory domain of CBS contains a tandem repeat of two CBS domains of β-α-β-β-α, a secondary structure motif found in other proteins. CBS has a C-terminal inhibitory domain. The C-terminal domain of cystathionine β-synthase regulates its activity via both intrasteric and allosteric effects and is important for maintaining the tetrameric state of the protein. This inhibition is alleviated by binding of the allosteric effector, adoMet
S-Adenosyl methionine
S-Adenosyl methionine is a common cosubstrate involved in methyl group transfers. SAM was first discovered in Italy by G. L. Cantoni in 1952. It is made from adenosine triphosphate and methionine by methionine adenosyltransferase . Transmethylation, transsulfuration, and aminopropylation are the...
, or by deletion of the regulatory domain; however, the magnitude of the effects differ. Mutations in this domain are correlated with hereditary diseases.
The heme domain contains an N-terminal loop that binds heme and provides the axial ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s C52 and H65. The distance of heme from the PLP
Pyridoxal-phosphate
Pyridoxal-phosphate is a prosthetic group of some enzymes. It is the active form of vitamin B6, which comprises three natural organic compounds, pyridoxal, pyridoxamine and pyridoxine.-Role as a coenzyme:...
binding site suggests its non-role in catalysis, however deletion of the heme domain causes loss of redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
sensitivity, therefore it is hypothesized that heme is a redox sensor. The presence of protoporphyrin IX in CBS is a unique PLP-dependent enzyme and is only found in the mammalian CBS. D. melanogaster
Drosophila melanogaster
Drosophila melanogaster is a species of Diptera, or the order of flies, in the family Drosophilidae. The species is known generally as the common fruit fly or vinegar fly. Starting from Charles W...
and D. discoides have truncated N-terminal extensions and therefore prevent the conserved histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
and cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
heme ligand residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
s. However, the Anopheles gambiae
Anopheles gambiae
Anopheles gambiae is a complex of at least seven morphologically distinguishable species of mosquitoes in the genus Anopheles. This complex was recognised in the 1960s and includes the most important vectors of malaria in sub-Saharan Africa and the most efficient malaria vectors known.This species...
sequence has a longer N-terminal extension than the human enzyme and contains the conserved histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
and cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
ligand residues like the human heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
. Therefore, it is possible that CBS in slime molds and insects are hemeproteins that suggest that the heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
domain is an early evolutionary innovation that arose before the separation of animals and the slime molds. The PLP
Pyridoxal-phosphate
Pyridoxal-phosphate is a prosthetic group of some enzymes. It is the active form of vitamin B6, which comprises three natural organic compounds, pyridoxal, pyridoxamine and pyridoxine.-Role as a coenzyme:...
is an internal aldimine
Aldimine
In organic chemistry, an aldimine is an imine that is an analog of an aldehyde.As such, aldimines have the general formula R–CH=N–R. Aldimines are similar to ketimines, which are analogs of ketones.An important subset of aldimines are the Schiff bases,...
and forms a Schiff base
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
with K119 in the active site. Between the catalytic and regulatory domains exists a hypersensitive site that causes proteolytic cleavage and produces a truncated dimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
ic enzyme that is more active than the original enzyme. Both truncated enzyme and the enzyme found in yeast are not regulated by adoMet. The yeast enzyme is also activated by the deletion of the C-terminal to produce the dimeric enzyme.
As of late 2007, two structures
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes and .
Enzymatic activity

Homocysteine
Homocysteine is a non-protein amino acid with the formula HSCH2CH2CHCO2H. It is a homologue of the amino acid cysteine, differing by an additional methylene group. It is biosynthesized from methionine by the removal of its terminal Cε methyl group...
to cystathionine
Cystathionine
Cystathionine is an intermediate in the synthesis of cysteine.It is generated from homocysteine and serine by cystathionine beta synthase.It is cleaved into cysteine and α-ketobutyrate by cystathionine gamma-lyase....
, which cystathione gamma lyase converts to cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
.
CBS occupies a pivotal position in mammalian sulfur metabolism at the homocysteine
Homocysteine
Homocysteine is a non-protein amino acid with the formula HSCH2CH2CHCO2H. It is a homologue of the amino acid cysteine, differing by an additional methylene group. It is biosynthesized from methionine by the removal of its terminal Cε methyl group...
junction where the decision to conserve methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
or to convert it to cysteine via the transsulfuration pathway
Transsulfuration pathway
The transsulfuration pathway is a metabolic pathway involving the interconversion of cysteine and homocysteine, through the intermediate cystathionine. In eukarotes, such as humans, the transsulfuration pathway is critical for creating cysteine from the essential amino acid methionine...
, is made. Moreover, the transsulfuration pathway is the only pathway capable of removing sulfur-containing amino acids under conditions of excess.
In analogy with other β-replacement enzymes, the reaction catalyzed by CBS is predicted to involve a series of adoMet-bound intermediates. Addition of serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
results in a transchiffization
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
reaction, which forms of an external aldimine
Aldimine
In organic chemistry, an aldimine is an imine that is an analog of an aldehyde.As such, aldimines have the general formula R–CH=N–R. Aldimines are similar to ketimines, which are analogs of ketones.An important subset of aldimines are the Schiff bases,...
. The aldimine
Aldimine
In organic chemistry, an aldimine is an imine that is an analog of an aldehyde.As such, aldimines have the general formula R–CH=N–R. Aldimines are similar to ketimines, which are analogs of ketones.An important subset of aldimines are the Schiff bases,...
undergoes proton abstraction at the α-carbon followed by elimination to generate an amino-acrylate
Acrylate
The acrylate ion is the ion of acrylic acid.Acrylates are the salts and esters of acrylic acid. They are also known as propenoates ....
intermediate. Nucleophilic attack by the thiolate of homocysteine on the aminoacrylate and reprotonation at Cα generate the external aldimine of cystathionine
Cystathionine
Cystathionine is an intermediate in the synthesis of cysteine.It is generated from homocysteine and serine by cystathionine beta synthase.It is cleaved into cysteine and α-ketobutyrate by cystathionine gamma-lyase....
. A final transaldimination reaction releases the final product, cystathionine. The final product, L-cystathionine can also form an aminoacrylate intermediate, indicating that the entire reaction of CBS is reversible.
The measured V0 of an enzyme-catalyzed reaction, in general, reflects the steady state (where [ES] is constant), even though V0 is limited to the early part of a reaction, and analysis of these initial rates is referred to as steady-state kinetics. Steady-state kinetic analysis of yeast CBS yields parallel lines. These results agree with the proposed ping-pong mechanism in which serine binding and release of water are followed by homocysteine binding and release of cystathionine. In contrast, the steady-state enzyme kinetics
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
of rat CBS yields intersecting lines, indicating that the β-substitutent of serine is not released from the enzyme prior to binding of homocysteine.
One of the alternate reactions involving CBS is the condensation of cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
with homocysteine to form cystathionine and hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
(H2S). H2S in the brain is produced from L-cysteine by CBS. This alternative metabolic pathway is also dependent on adoMet.
CBS enzyme activity is not found in all tissues and cells. It is absent from heart, lung, testes, adrenal, and spleen in rats. In humans, it has been shown to be absent in heart muscle and primary cultures of human aortic endothelial cells. The lack of CBS in these tissues implies that these tissues are unable to synthesize cysteine and that cysteine must be supplied from extracellular sources. It also suggests that these tissues might have increased sensitivity to homocysteine toxicity because they cannot catabolize excess homocysteine via transsulfuration.
Regulation
Allosteric activation of CBS adoMet determines the metabolic fate of L-homocysteine. Mammalian CBS is activated 2.5-5-fold by adoMet with a dissociation constant of 15 µM. adoMet is an allosteric activator that increases the Vmax of the CBS reaction but does not affect the Kms for the substrates. In other words, adoMet stimulates CBS activity by increasing the turnover rate rather than the binding of substrates to the enzyme.Human CBS performs a crucial step in the biosynthetic pathway of cysteine by providing a regulatory control point for adoMet . L-homocysteine, after being methylated to methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
, can be converted to adoMet , which donates methyl groups to a variety of substrates, e.g., neurotransmitters, proteins, and nucleic acids. adoMet functions as an allosteric activator of CBS and exerts control on its biosynthesis: low concentrations of adoMet result in low CBS activity, thereby funneling homocysteine in the transmethylation pathway toward adoMet formation. Methionine is transmethylated to homocysteine via S-adenosyl-methionine (SAM). In contrast, high adoMet concentrations allow the clearance of homocysteine into the transsulfuration pathway, leading to cysteine biosynthesis.
In mammals, CBS is a highly regulated enzyme, which contains a heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
cofactor that functions as a redox sensor, that can modulate its activity in response to changes in the redox potential. If the resting form of CBS in the cell has ferrous heme, the potential exists for activating the enzyme under oxidizing conditions by conversion to the ferric state. The Fe (II) form of the enzyme is inhibited upon binding CO or nitric oxide, whereas enzyme activity is doubled when the Fe (II) is oxidized to Fe (III). The redox state of the heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
is pH dependent, with oxidation of Fe (II)-CBS to Fe (III)-CBS being favored at low pH conditions.
Since mammalian CBS contains a heme cofactor, whereas yeast and protozoan enzyme from Trypanosoma cruzi do not have heme cofactors, researchers have speculated that heme is not required for CBS activity.
CBS is regulated at the transcriptional level by NF-Y, SP-1, and SP-3. In addition it is upregulated transcriptionally by glucocorticoids and glucogen, and downregulated by insulin. Methionine upregulates CBS at the post-transcriptional level.
Human disease
Down syndromeDown syndrome
Down syndrome, or Down's syndrome, trisomy 21, is a chromosomal condition caused by the presence of all or part of an extra 21st chromosome. It is named after John Langdon Down, the British physician who described the syndrome in 1866. The condition was clinically described earlier in the 19th...
is a medical condition characterized by an overexpression of cystathionine beta synthase (CBS) and a low level of homocysteine in the blood.
It has been speculated that cystathionine beta synthase overexpression could be the major culprit in this disease (along with dysfunctioning of GabaA and Dyrk1a). The phenotype of down syndrome is the opposite of Hyperhomocysteinemia (described below). Pharmacologicals inhibitors of CBS have been patented by the Jerome Lejeune Foundation (november 2011) and trials (animals and humans are planned).
Hyperhomocysteinemia
Hyperhomocysteinemia
Hyperhomocysteinemia or hyperhomocysteinaemia is a medical condition characterized by an abnormally large level of homocysteine in the blood....
is a medical condition characterized by an abnormally large level of homocysteine
Homocysteine
Homocysteine is a non-protein amino acid with the formula HSCH2CH2CHCO2H. It is a homologue of the amino acid cysteine, differing by an additional methylene group. It is biosynthesized from methionine by the removal of its terminal Cε methyl group...
in the blood. Mutations in CBS are the single most common cause of hereditary hyperhomocysteinemia. Inborn errors in CBS result in hyperhomocysteinemia
Hyperhomocysteinemia
Hyperhomocysteinemia or hyperhomocysteinaemia is a medical condition characterized by an abnormally large level of homocysteine in the blood....
with complications in the cardiovascular system leading to early and aggressive arterial disease. Hyperhomocysteinemia
Hyperhomocysteinemia
Hyperhomocysteinemia or hyperhomocysteinaemia is a medical condition characterized by an abnormally large level of homocysteine in the blood....
also affects three other major organ systems including the ocular, central nervous, and skeletal.
Homocystinuria
Homocystinuria
Homocystinuria, also known as cystathionine beta synthase deficiency or CBS deficiency, is an inherited disorder of the metabolism of the amino acid methionine, often involving cystathionine beta synthase...
due to CBS deficiency is a special type of hyperhomocysteinemia. It is a rare, hereditary recessive autosomal disease, in general, diagnosed during childhood. A total of 131 different homocystinuria-causing mutations have been identified. A common functional feature of the mutations in the CBS domains is that the mutations abolish or strongly reduce activation by adoMet. No specific cure has been discovered for homocystinuria; however, many people are treated using high doses of vitamin B6
Vitamin B6
Vitamin B6 is a water-soluble vitamin and is part of the vitamin B complex group. Several forms of the vitamin are known, but pyridoxal phosphate is the active form and is a cofactor in many reactions of amino acid metabolism, including transamination, deamination, and decarboxylation...
, which is a cofactor of CBS.
Bioengineering
Cystathionine beta synthase (CBS) is involved in oocyteOocyte
An oocyte, ovocyte, or rarely ocyte, is a female gametocyte or germ cell involved in reproduction. In other words, it is an immature ovum, or egg cell. An oocyte is produced in the ovary during female gametogenesis. The female germ cells produce a primordial germ cell which undergoes a mitotic...
development. However, little is known about the regional and cellular expression patterns of CBS in the ovary and research is now focused on determining the location and expression during follicle development in the ovaries.
See also
- HomocystinuriaHomocystinuriaHomocystinuria, also known as cystathionine beta synthase deficiency or CBS deficiency, is an inherited disorder of the metabolism of the amino acid methionine, often involving cystathionine beta synthase...
- CysteineCysteineCysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
- MetabolismMetabolismMetabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
- Amino Acids
- S-Adenosyl-L-methionine
- HemeHemeA heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...

