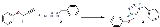
Azide alkyne Huisgen cycloaddition
Encyclopedia
The Azide-Alkyne Huisgen Cycloaddition is a 1,3-dipolar cycloaddition
between an azide
and a terminal or internal alkyne
to give a 1,2,3-triazole
. Rolf Huisgen
was the first to understand the scope of this organic reaction
. American chemist
K. Barry Sharpless
has referred to this cycloaddition
as "the cream of the crop" of click chemistry
.
 In the reaction above azide 2 reacts neatly with alkyne 1 to afford the triazole 3 as a mixture of 1,4-adduct and 1,5-adduct at 98 °C in 18 hours.
In the reaction above azide 2 reacts neatly with alkyne 1 to afford the triazole 3 as a mixture of 1,4-adduct and 1,5-adduct at 98 °C in 18 hours.
The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene dipolarophiles has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes.
Although azides are not the most reactive 1,3-dipole available for reaction, they are preferred for their relative lack of side reactions and stability in typical synthetic conditions.
at the Scripps Research Institute
.
While the copper(I) catalyzed variant gives rise to a triazole from a terminal alkyne and an azide, formally it is not a 1,3-dipolar cycloaddition and thus should not be termed a Huisgen cycloaddition. This reaction is better termed the Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC).
While the reaction can be performed using commercial sources of copper(I) such as cuprous bromide or iodide, the reaction works much better using a mixture of copper(II) (e.g. copper(II) sulfate) and a reducing agent (e.g. sodium ascorbate) to produce Cu(I) in situ. As Cu(I) is unstable in aqueous solvents, stabilizing ligands are effective for improving the reaction outcome, especially if tris-(benzyltriazolylmethyl)amine (TBTA) is used. The reaction can be run in a variety of solvents, and mixtures of water and a variety of (partially) miscible organic solvents including alcohols, DMSO, DMF, tBuOH and acetone. Owing to the powerful coordinating ability of nitriles towards Cu(I), it is best to avoid acetonitrile as the solvent. The starting reagents need not be completely soluble for the reaction to be successful. In many cases, the product can be simple filtered from the solution as the only purification step required.
NH-1,2,3-triazoles are also prepared from alkynes in a sequence called the Banert cascade
.
The utility of the Cu(I) catalyzed click reaction has also been demonstrated in the polymerization
reaction of a bis-azide and a bis-alkyne with copper(I) and TBTA to a conjugated
fluorene
based polymer
. The degree of polymerization
easily exceeds 50. With a stopper molecule such as phenyl azide
, well-defined phenyl end-group
s are obtained.
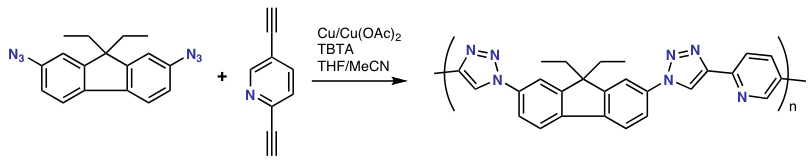 The copper mediated azide-alkyne cycloaddition is receiving widespread use in material and surface sciences. Most variations in coupling polymers with other polymers or small molecules have been explored. Current shortcomings are that the terminal alkyne appears to participate in free radical polymerizations. This requires protection of the terminal alkyne with a trimethyl silyl protecting group
The copper mediated azide-alkyne cycloaddition is receiving widespread use in material and surface sciences. Most variations in coupling polymers with other polymers or small molecules have been explored. Current shortcomings are that the terminal alkyne appears to participate in free radical polymerizations. This requires protection of the terminal alkyne with a trimethyl silyl protecting group
and subsequent deprotection after the radical reaction are completed. Similarly the use of organic solvents, copper (I) and inert atmospheres to do the cycloaddition with many polymers makes the "click" label inappropriate for such reactions. An aqueous protocol for performing the cycloaddition with free radical polymers is highly desirable.
The CuAAC click reaction also effectively couples polystyrene
and bovine serum albumin
(BSA). The result is an amphiphilic biohybrid. BSA contains a thiol
group at Cys
-34 which is functionalized with an alkyne
group. In water the biohybrid micelle
s with a diameter
of 30 to 70 nanometer form aggregates.
in the 1970s, which he ran at elevated temperatures.. The traditional reaction is slow and thus requires high temperatures. However, the azides and alkynes are both kinetically stable.
As mentioned above, copper-catalysed click reactions work essentially on terminal alkynes. The Cu species undergo metal insertion reaction into the terminal alkynes. The Cu(I) species may either be introduced as preformed complexes, or are otherwise generated in the reaction pot itself by one of the following ways:
Commonly used solvents are polar aprotic solvents such as THF
, DMSO
, Acetonitrile
, DMF
as well as in non-polar aprotic solvents such as toluene
. Neat solvents or a mixture of solvents may be used.
DIPEA (N,N-Diisopropylethylamine) and Et3N (triethylamine) are commonly used bases.
calculations. Copper is a 1st row transition metal
. It has the electronic configuration [Ar] 3d10 4s1. The copper (I) species generated in situ forms a pi complex with the triple bond of a terminal alkyne. In the presence of a base, the terminal hydrogen, being the most acidic is deprotonated first to give a Cu acetylide intermediate. Studies have shown that the reaction is second order with respect to Cu. It has been suggested that the transition state involves two copper atoms. One copper atom is bonded to the acetylide while the other Cu atom serves to activate the azide. The metal center coordinates with the electrons on the nitrogen atom. The azide and the acetylide are not coordinated to the same Cu atom in this case. The ligands employed are labile and are weakly coordinating. The azide displaces one ligand to generate a copper-azide-acetylide complex . At this point cyclisation takes place. This is followed by protonation
; the source of proton being the hydrogen which was pulled off from the terminal acetylene by the base. The product is formed by dissociation and the catalyst ligand complex is regenerated for further reaction cycles.
The reaction is assisted by the copper, which, when coordinated with the acetylide lowers the pKa of the alkyne C-H by up to 9.8 units. Thus under certain conditions, the reaction may be carried out even in the absence of a base.
In the uncatalysed reaction the alkyne remains a poor electrophile. Thus high energy barriers lead to slow reaction rates.
s employed are usually labile i.e. they can be displaced easily. Though the ligand plays no direct role in the reaction the presence of a ligand has its advantages.
The ligand protects the Cu ion from interactions leading to degradation and formation of side products and also prevents the oxidation of the Cu(I) species to the Cu(II). Furthermore, the ligand functions as a proton acceptor thus eliminating the need of a base.
-catalysed 1,3-dipolar azide-alkyne cycloaddition (RuAAC) gives the 1,5-triazole.
Unlike CuAAC in which only terminal alkynes reacted, in RuAAC both, terminal and internal alkynes can participate in the reaction. This suggests that ruthenium acetylides are not involved in the catalytic cycle
.
The proposed mechanism suggests that in the first step, the spectator ligands undergo displacement reaction to produce an activated complex
which is converted, via oxidative coupling of an alkyne and an azide to the ruthenium containing metallocyle (Ruthenacycle). The new C-N bond
is formed between the more electronegative and less sterically-demanding carbon of the alkyne and the terminal nitrogen of the azide. The metallacycle intermediate then undergoes reductive elimination releasing the aromatic triazole product and regenerating the catalyst or the activated complex for further reaction cycles.
Cp*RuCl(PPh3)2, Cp*Ru(COD)and Cp*[RuCl4] are commonly used ruthenium catalysts. Catalysts containing cyclopentadienyl(Cp) group are also used. However, better results are observed with the pentamethylcyclopentadienyl(Cp*) version. This may be due to the sterically demanding Cp* group which facilitates the displacement of the spectator ligands.
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
between an azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
and a terminal or internal alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
to give a 1,2,3-triazole
1,2,3-Triazole
1,2,3-Triazole is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms...
. Rolf Huisgen
Rolf Huisgen
Rolf Huisgen is a German chemist. He was born in Gerolstein and studied in Munich under the supervision of Heinrich Otto Wieland. After completing his Ph.D. in 1943 and his habilitation in 1947, he became professor at the University of Tübingen in 1949...
was the first to understand the scope of this organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
. American chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
K. Barry Sharpless
K. Barry Sharpless
Karl Barry Sharpless is an American chemist known for his work on stereoselective reactions.-Early years:Sharpless was born in Philadelphia. He graduated from Friends' Central School in 1959. He continued his studies at Dartmouth College and earned his Ph.D from Stanford University in 1968...
has referred to this cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
as "the cream of the crop" of click chemistry
Click chemistry
Click chemistry is a chemical philosophy introduced by K. Barry Sharpless of The Scripps Research Institute, in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together...
.

The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene dipolarophiles has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes.
Although azides are not the most reactive 1,3-dipole available for reaction, they are preferred for their relative lack of side reactions and stability in typical synthetic conditions.
Copper catalysis
A notable variant of the Huisgen 1,3-dipolar cycloaddition is the copper(I) catalyzed variant, no longer a true concerted cycloaddition, in which organic azides and terminal alkynes are united to afford 1,4-regioisomers of 1,2,3-triazoles as sole products (substitution at positions 1' and 4' as shown above). The copper(I)-catalyzed variant was first reported in 2002 in independent publications by by Morten Meldal at the Carlsberg Laboratory in Denmark and Valery Fokin and K. Barry SharplessK. Barry Sharpless
Karl Barry Sharpless is an American chemist known for his work on stereoselective reactions.-Early years:Sharpless was born in Philadelphia. He graduated from Friends' Central School in 1959. He continued his studies at Dartmouth College and earned his Ph.D from Stanford University in 1968...
at the Scripps Research Institute
The Scripps Research Institute
The Scripps Research Institute is an American medical research facility that focuses on research in the basic biomedical sciences. Headquartered in La Jolla, California, with a sister facility in Jupiter, Florida, the institute is home to 3,000 scientists, technicians, graduate students, and...
.
While the copper(I) catalyzed variant gives rise to a triazole from a terminal alkyne and an azide, formally it is not a 1,3-dipolar cycloaddition and thus should not be termed a Huisgen cycloaddition. This reaction is better termed the Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC).
While the reaction can be performed using commercial sources of copper(I) such as cuprous bromide or iodide, the reaction works much better using a mixture of copper(II) (e.g. copper(II) sulfate) and a reducing agent (e.g. sodium ascorbate) to produce Cu(I) in situ. As Cu(I) is unstable in aqueous solvents, stabilizing ligands are effective for improving the reaction outcome, especially if tris-(benzyltriazolylmethyl)amine (TBTA) is used. The reaction can be run in a variety of solvents, and mixtures of water and a variety of (partially) miscible organic solvents including alcohols, DMSO, DMF, tBuOH and acetone. Owing to the powerful coordinating ability of nitriles towards Cu(I), it is best to avoid acetonitrile as the solvent. The starting reagents need not be completely soluble for the reaction to be successful. In many cases, the product can be simple filtered from the solution as the only purification step required.
NH-1,2,3-triazoles are also prepared from alkynes in a sequence called the Banert cascade
Banert cascade
The Banert cascade is an organic reaction in which an NH-1,2,3-triazole is prepared from a propargyl halide or sulfate and sodium azide in a dioxane- water mixture at elevated temperatures...
.
The utility of the Cu(I) catalyzed click reaction has also been demonstrated in the polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
reaction of a bis-azide and a bis-alkyne with copper(I) and TBTA to a conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
fluorene
Fluorene
Fluorene, or 9H-fluorene, is a polycyclic aromatic hydrocarbon. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It is combustible. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar...
based polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
. The degree of polymerization
Degree of polymerization
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.For a homopolymer, there is only one type of monomeric unit andthe number-average degree of polymerization is given by...
easily exceeds 50. With a stopper molecule such as phenyl azide
Phenyl azide
Phenylazide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It has a pungent odor. The structure consists of a linear azide substituent bound to a phenyl group...
, well-defined phenyl end-group
End-group
An end-group in polymer chemistry is a constitutional unit that is an extremity of a macromolecule or oligomer molecule. For example the end-group of a PET polyester may be an alcohol group or a carboxylic acid group...
s are obtained.

Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
and subsequent deprotection after the radical reaction are completed. Similarly the use of organic solvents, copper (I) and inert atmospheres to do the cycloaddition with many polymers makes the "click" label inappropriate for such reactions. An aqueous protocol for performing the cycloaddition with free radical polymers is highly desirable.
The CuAAC click reaction also effectively couples polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
and bovine serum albumin
Bovine serum albumin
Bovine serum albumin is a serum albumin protein derived from cows. It is often used as a protein concentration standard....
(BSA). The result is an amphiphilic biohybrid. BSA contains a thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
group at Cys
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
-34 which is functionalized with an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
group. In water the biohybrid micelle
Micelle
A micelle is an aggregate of surfactant molecules dispersed in a liquid colloid. A typical micelle in aqueous solution forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single tail regions in the micelle centre. This phase is...
s with a diameter
Diameter
In geometry, a diameter of a circle is any straight line segment that passes through the center of the circle and whose endpoints are on the circle. The diameters are the longest chords of the circle...
of 30 to 70 nanometer form aggregates.
Copper Catalysts
The use of a Cu catalyst in water was an improvement over the same reaction first popularized by Rolf HuisgenRolf Huisgen
Rolf Huisgen is a German chemist. He was born in Gerolstein and studied in Munich under the supervision of Heinrich Otto Wieland. After completing his Ph.D. in 1943 and his habilitation in 1947, he became professor at the University of Tübingen in 1949...
in the 1970s, which he ran at elevated temperatures.. The traditional reaction is slow and thus requires high temperatures. However, the azides and alkynes are both kinetically stable.
As mentioned above, copper-catalysed click reactions work essentially on terminal alkynes. The Cu species undergo metal insertion reaction into the terminal alkynes. The Cu(I) species may either be introduced as preformed complexes, or are otherwise generated in the reaction pot itself by one of the following ways:
- A Cu compound (in which copper is present in the +2 oxidation state) is added to the reaction in presence of a reducing agent which reduces the Cu from the (+2) to the (+1) oxidation state. The advantage of generating the Cu(I) species in this manner is it eliminates the need of a base in the reaction. Also the presence of reducing agent makes up for any oxygen which may have gotten into the system. Oxygen oxidises the Cu(I) to Cu(II) which impedes the reaction and results in low yields. One of the more commonly-used Cu compounds is CuSO4
- Oxidation of Cu(0) metal
- Halides of copper may be used where solubility is an issue. However, the iodide and bromide Cu salts require either the presence of amines or higher temperatures.
Commonly used solvents are polar aprotic solvents such as THF
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
, DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, Acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
, DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
as well as in non-polar aprotic solvents such as toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
. Neat solvents or a mixture of solvents may be used.
DIPEA (N,N-Diisopropylethylamine) and Et3N (triethylamine) are commonly used bases.
Mechanism
A mechanism for the reaction has been suggested based on density functional theoryDensity functional theory
Density functional theory is a quantum mechanical modelling method used in physics and chemistry to investigate the electronic structure of many-body systems, in particular atoms, molecules, and the condensed phases. With this theory, the properties of a many-electron system can be determined by...
calculations. Copper is a 1st row transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
. It has the electronic configuration [Ar] 3d10 4s1. The copper (I) species generated in situ forms a pi complex with the triple bond of a terminal alkyne. In the presence of a base, the terminal hydrogen, being the most acidic is deprotonated first to give a Cu acetylide intermediate. Studies have shown that the reaction is second order with respect to Cu. It has been suggested that the transition state involves two copper atoms. One copper atom is bonded to the acetylide while the other Cu atom serves to activate the azide. The metal center coordinates with the electrons on the nitrogen atom. The azide and the acetylide are not coordinated to the same Cu atom in this case. The ligands employed are labile and are weakly coordinating. The azide displaces one ligand to generate a copper-azide-acetylide complex . At this point cyclisation takes place. This is followed by protonation
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
; the source of proton being the hydrogen which was pulled off from the terminal acetylene by the base. The product is formed by dissociation and the catalyst ligand complex is regenerated for further reaction cycles.
The reaction is assisted by the copper, which, when coordinated with the acetylide lowers the pKa of the alkyne C-H by up to 9.8 units. Thus under certain conditions, the reaction may be carried out even in the absence of a base.
In the uncatalysed reaction the alkyne remains a poor electrophile. Thus high energy barriers lead to slow reaction rates.
Ligand assistance
The ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s employed are usually labile i.e. they can be displaced easily. Though the ligand plays no direct role in the reaction the presence of a ligand has its advantages.
The ligand protects the Cu ion from interactions leading to degradation and formation of side products and also prevents the oxidation of the Cu(I) species to the Cu(II). Furthermore, the ligand functions as a proton acceptor thus eliminating the need of a base.
Ruthenium catalysis
The rutheniumRuthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
-catalysed 1,3-dipolar azide-alkyne cycloaddition (RuAAC) gives the 1,5-triazole.
Unlike CuAAC in which only terminal alkynes reacted, in RuAAC both, terminal and internal alkynes can participate in the reaction. This suggests that ruthenium acetylides are not involved in the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
.
The proposed mechanism suggests that in the first step, the spectator ligands undergo displacement reaction to produce an activated complex
Activated complex
In chemistry an activated complex is defined by the International Union of Pure and Applied Chemistry as "that assembly of atoms which corresponds to an arbitrary infinitesimally small region at or near the col of a potential energy surface"...
which is converted, via oxidative coupling of an alkyne and an azide to the ruthenium containing metallocyle (Ruthenacycle). The new C-N bond
Carbon-nitrogen bond
A carbon–nitrogen bond is a covalent bond between carbon and nitrogen and is one of the most abundant bonds in organic chemistry and biochemistry....
is formed between the more electronegative and less sterically-demanding carbon of the alkyne and the terminal nitrogen of the azide. The metallacycle intermediate then undergoes reductive elimination releasing the aromatic triazole product and regenerating the catalyst or the activated complex for further reaction cycles.
Cp*RuCl(PPh3)2, Cp*Ru(COD)and Cp*[RuCl4] are commonly used ruthenium catalysts. Catalysts containing cyclopentadienyl(Cp) group are also used. However, better results are observed with the pentamethylcyclopentadienyl(Cp*) version. This may be due to the sterically demanding Cp* group which facilitates the displacement of the spectator ligands.
External links
- http://clickchemicals.com Click Chemicals. A new site all about click chemistry featuring in depth discussion, faq's and links to key papers.

