
Auger electron spectroscopy
Encyclopedia
Auger electron spectroscopy (AES; pronounced oʒe in French) is a common analytical technique used specifically in the study of surfaces
and, more generally, in the area of materials science
. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom
after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner
and Pierre Auger
in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik
in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy
data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy
, gas-phase chemistry, and throughout the microelectronics
industry.
to 50 keV, a core state electron can be removed leaving behind a hole. As this is an unstable state, the core hole can be filled by an outer shell electron, whereby the electron moving to the lower energy level loses an amount of energy equal to the difference in orbital energies. The transition energy can be coupled to a second outer shell electron which will be emitted from the atom if the transferred energy is greater than the orbital binding energy. An emitted electron will have a kinetic energy of:

where ,
, 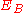 ,
, 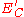 are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations. Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations. Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
The types of state-to-state transitions available to electrons during an Auger event are dependent on several factors, ranging from initial excitation energy to relative interaction rates, yet are often dominated by a few characteristic transitions. Because of the interaction between an electron's spin and orbital angular momentum (spin-orbit coupling) and the concomitant energy level splitting for various shells in an atom, there are a variety of transition pathways for filling a core hole. Energy levels are labeled using a number of different schemes such as the j-j coupling method for heavy elements (Z ≥ 75), the Russell-Saunders L-S method for lighter elements (Z < 20), and a combination of both for intermediate elements. The j-j coupling method, which is historically linked to X-ray notation
, is almost always used to denote Auger transitions. Thus for a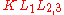 transition, K represents the core level hole,
transition, K represents the core level hole, 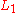 the relaxing electron's initial state, and
the relaxing electron's initial state, and 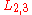 the emitted electron's initial energy state. Figure 1(b) illustrates this transition with the corresponding spectroscopic notation. The energy level of the core hole will often determine which transition types will be favored. For single energy levels, i.e. K, transitions can occur from the L levels, giving rise to strong KLL type peaks in an Auger spectrum. Higher level transitions can also occur, but are less probable. For multi-level shells, transitions are available from higher energy orbitals (different n, ℓ quantum numbers) or energy levels within the same shell (same n, different ℓ number). The result are transitions of the type LMM and KLL along with faster Coster–Kronig transitions such as LLM. while Coster–Kronig transitions are faster, they are also less energetic and thus harder to locate on an Auger spectrum. As the atomic number
the emitted electron's initial energy state. Figure 1(b) illustrates this transition with the corresponding spectroscopic notation. The energy level of the core hole will often determine which transition types will be favored. For single energy levels, i.e. K, transitions can occur from the L levels, giving rise to strong KLL type peaks in an Auger spectrum. Higher level transitions can also occur, but are less probable. For multi-level shells, transitions are available from higher energy orbitals (different n, ℓ quantum numbers) or energy levels within the same shell (same n, different ℓ number). The result are transitions of the type LMM and KLL along with faster Coster–Kronig transitions such as LLM. while Coster–Kronig transitions are faster, they are also less energetic and thus harder to locate on an Auger spectrum. As the atomic number
Z increases, so too does the number of potential Auger transitions. Fortunately, the strongest electron-electron interactions are between levels which are close together, giving rise to characteristic peaks in an Auger spectrum. KLL and LMM peaks are some of the most commonly identified transitions during surface analysis. Finally, valence band electrons can also fill core holes or be emitted during KVV-type transitions.
Several models, both phenomenological and analytical, have been developed to describe the energetics of Auger transitions. One of the most tractable descriptions, put forth by Jenkins and Chung, estimates the energy of Auger transition ABC as:
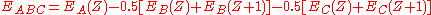
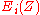 are the binding energies of the
are the binding energies of the 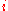 th level in element of atomic number Z and
th level in element of atomic number Z and 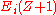 are the energies of the same levels in the next element up in the periodic table. While useful in practice, a more rigorous model accounting for effects such as screening and relaxation probabilities between energy levels gives the Auger energy as:
are the energies of the same levels in the next element up in the periodic table. While useful in practice, a more rigorous model accounting for effects such as screening and relaxation probabilities between energy levels gives the Auger energy as:
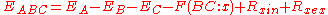
where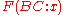 is the energy of interaction between the B and C level holes in a final atomic state x and the Rs represent intra- and extra-atomic transition energies accounting for electronic screening. Auger electron energies can be calculated based on measured values of the various
is the energy of interaction between the B and C level holes in a final atomic state x and the Rs represent intra- and extra-atomic transition energies accounting for electronic screening. Auger electron energies can be calculated based on measured values of the various 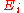 and compared to peaks in the secondary electron spectrum in order to identify chemical species. This technique has been used to compile several reference databases used for analysis in current AES setups.
and compared to peaks in the secondary electron spectrum in order to identify chemical species. This technique has been used to compile several reference databases used for analysis in current AES setups.
in a solid. The escape depth of electrons is therefore localized to within a few nanometers of the target surface, giving AES an extreme sensitivity to surface species. Because of the low energy of Auger electrons, most AES setups are run under ultra-high vacuum (UHV) conditions. Such measures prevent electron scattering off of residual gas atoms as well as the formation of a thin "gas (adsorbate) layer" on the surface of the specimen which degrades analytical performance. A typical AES setup is shown schematically in figure 2. In this configuration, focused electrons are incident on a sample and emitted electrons are deflected into a cylindrical mirror analyzer (CMA). In the detection unit, Auger electrons are multiplied and the signal sent to data processing electronics. Collected Auger electrons are plotted as a function of energy against the broad secondary electron background spectrum.
Since the intensity of the Auger peaks may be small compared to the noise level of the background, AES is often run in a derivative mode which serves to highlight the peaks by modulating the electron collection current via a small applied AC voltage. Since this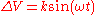 , the collection current becomes
, the collection current becomes 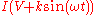 . Taylor expanding
. Taylor expanding
gives:
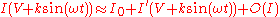
Using the setup in figure 2, detecting the signal at frequency ω will give a value for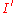 or
or  . Plotting in derivative mode also emphasizes Auger fine structure which appear as small secondary peaks surrounding the primary Auger peak. These secondary peaks, not to be confused with high energy satellites which are discussed later, arise from the presence of the same element in multiple different chemical states on a surface (i.e. Adsorbate layers) or from relaxation transitions involving valence band electrons of the substrate. Figure 3 illustrates a derivative spectrum from a copper nitride film clearly showing the Auger peaks. The peak in derivative mode is not the true Auger peak, but rather the point of maximum slope of N(E), but this concern is usually ignored.
. Plotting in derivative mode also emphasizes Auger fine structure which appear as small secondary peaks surrounding the primary Auger peak. These secondary peaks, not to be confused with high energy satellites which are discussed later, arise from the presence of the same element in multiple different chemical states on a surface (i.e. Adsorbate layers) or from relaxation transitions involving valence band electrons of the substrate. Figure 3 illustrates a derivative spectrum from a copper nitride film clearly showing the Auger peaks. The peak in derivative mode is not the true Auger peak, but rather the point of maximum slope of N(E), but this concern is usually ignored.
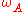 , is thus related to the fluorescence
, is thus related to the fluorescence
(x-ray) yield,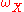 , by the relation,
, by the relation,
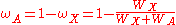
where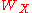 is the X-ray transition probability and
is the X-ray transition probability and 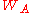 is the Auger transition probability. Attempts to relate the fluorescence and Auger yields to atomic number have resulted in plots similar to figure 4. A clear transition from electron to photon emission is evident in this chart for increasing atomic number. For heavier elements, x-ray yield becomes greater than Auger yield, indicating an increased difficulty in measuring the Auger peaks for large Z-values. Conversely, AES is sensitive to the lighter elements, and unlike X-ray fluorescence
is the Auger transition probability. Attempts to relate the fluorescence and Auger yields to atomic number have resulted in plots similar to figure 4. A clear transition from electron to photon emission is evident in this chart for increasing atomic number. For heavier elements, x-ray yield becomes greater than Auger yield, indicating an increased difficulty in measuring the Auger peaks for large Z-values. Conversely, AES is sensitive to the lighter elements, and unlike X-ray fluorescence
, Auger peaks can be detected for elements as light as lithium
(Z = 3). Lithium
represents the lower limit for AES sensitivity since the Auger effect is a "three state" event necessitating at least three electrons. Neither H
nor He
can be detected with this technique. For K-level based transitions, Auger effects are dominant for Z < 15 while for L- and M-level transitions, AES data can be measured for Z ≤ 50. The yield limits effectively prescribe a cutoff for AES sensitivity, but complex techniques can be utilized to identify heavier elements, such as uranium
and americium
, using the Auger effect.
Another critical quantity that determines yield of Auger electrons at a detector is the electron impact cross-section. Early approximations (in cm2) of the cross-section were based on the work of Worthington and Tomlin,
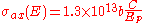
with b acting as a scaling factor between 0.25 and 0.35, and C a function of the primary electron beam energy,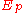 . While this value of
. While this value of 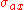 is calculated for an isolated atom, a simple modification can be made to account for matrix effects:
is calculated for an isolated atom, a simple modification can be made to account for matrix effects:

where α is the angle to the surface normal of the incident electron beam; rm can be established empirically and encompasses electron interactions with the matrix such as ionization due to backscattered electrons. Thus the total yield can be written as: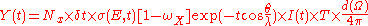
Here Nx is the number of x atoms per volume, λ the electron escape depth, θ the analyzer angle, T the transmission of the analyzer, I(t) the electron excitation flux at depth t, dΩ the solid angle, and δt is the thickness of the layer being probed. Encompassed in these terms, especially the Auger yield which is related to the transition probability, is the quantum mechanical overlap of the initial and final state wave functions. Precise expressions for the transition probability, based on first-order perturbation Hamiltonian
s, can be found in Thompson and Baker. Often, all of these terms are not known, so most analyses compare measured yields with external standards of known composition. Ratios of the acquired data to standards can eliminate common terms, especially experimental setup characteristics and material parameters, and can be used to determine element composition. Comparison techniques work best for samples of homogeneous binary materials or uniform surface layers, while elemental identification is best obtained from comparison of pure samples.
is sometimes used with Auger spectroscopy to perform depth profiling experiments. Sputtering removes thin outer layers of a surface so that AES can be used to determine the underlying composition. Depth profiles are shown as either Auger peak height vs. sputter time or atomic concentration vs. depth. Precise depth milling through sputtering has made profiling an invaluable technique for chemical analysis of nanostructured materials and thin films. AES is also used extensively as an evaluation tool on and off fab lines in the microelectronics industry, while the versatility and sensitivity of the Auger process makes it a standard analytical tool in research labs.
Despite the advantages of high spatial resolution and precise chemical sensitivity attributed to AES, there are several factors that can limit the applicability of this technique, especially when evaluating solid specimens. One of the most common limitations encountered with Auger spectroscopy are charging effects in non-conducting samples. Charging results when the number of secondary electrons leaving the sample is different to the number of incident electrons, giving rise to a net polarity at the surface. Both positive and negative surface charges severely alter the yield of electrons emitted from the sample and hence distort the measured Auger peaks. To complicate matters, neutralization methods employed in other surface analysis techniques, such as secondary ion mass spectrometry
(SIMS), are not applicable to AES, as these methods usually involve surface bombardment with either electrons or ions (i.e. flood gun
). Several processes have been developed to combat the issue of charging, though none of them is ideal and still make quantification of AES data difficult. One such technique involves depositing conductive pads near the analysis area to minimize regional charging. However, this type of approach limits SAM applications as well as the amount of sample material available for probing. A related technique involves thinning or "dimpling" a non-conductive layer with Ar+
ions and then mounting the sample to a conductive backing prior to AES. This method has been debated, with claims that the thinning process leaves elemental artifacts on a surface and/or creates damaged layers that distort bonding and promote chemical mixing in the sample. As a result, the compositional AES data is considered suspect. The most common setup to minimize charging effects includes use of a glancing angle (~10°) electron beam and a carefully tuned bombarding energy (between 1.5 keV and 3 keV). Control of both the angle and energy can subtly alter the number of emitted electrons vis-à-vis the incident electrons and thereby reduce or altogether eliminate sample charging.
In addition to charging effects, AES data can be obscured by the presence of characteristic energy losses in a sample and higher order atomic ionization events. Electrons ejected from a solid will generally undergo multiple scattering events and lose energy in the form of collective electron density oscillations called plasmons. If plasmon losses have energies near that of an Auger peak, the less intense Auger process may become dwarfed by the plasmon peak. As Auger spectra are normally weak and spread over many eV of energy, they are difficult to extract from the background and in the presence of plasmon losses, deconvolution of the two peaks becomes extremely difficult. For such spectra, additional analysis through chemical sensitive surface techniques like x-ray photoelectron spectroscopy
(XPS) is often required to disentangle the peaks. Sometimes an Auger spectrum can also exhibit "satellite" peaks at well-defined off-set energies from the parent peak. Origin of the satellites is usually attributed to multiple ionization events in an atom or ionization cascades in which a series of electrons is emitted as relaxation occurs for core holes of multiple levels. The presence of satellites can distort the true Auger peak and/or small peak shift information due to chemical bonding at the surface. Several studies have been undertaken to further quantify satellite peaks.
Despite these sometimes substantial drawbacks, Auger electron spectroscopy is a widely used surface analysis technique that has been successfully applied to many diverse fields ranging from gas phase chemistry to nanostructure characterization. Very new class of high-resolving electrostatic energy analyzers recently developed – the face-field analyzers (FFA) can be used for remote electron spectroscopy of distant surfaces or surfaces with large roughness or even with deep dimples. These instruments are designed as if to be specifically used in combined scanning electron microscope
s (SEMs). "FFA" in principle have no perceptible end-fields, which usually distort focusing in most of analysers known, for example, well known CMA.
Sensitivity, quantitative detail, and ease of use have brought AES from an obscure nuisance effect to a functional and practical characterization technique in just over fifty years. With applications both in the research laboratory and industrial settings, AES will continue to be a cornerstone of surface-sensitive electron-based spectroscopies.
Surface science
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid-gas interfaces. It includes the fields of surface chemistry and surface physics. Some related...
and, more generally, in the area of materials science
Materials science
Materials science is an interdisciplinary field applying the properties of matter to various areas of science and engineering. This scientific field investigates the relationship between the structure of materials at atomic or molecular scales and their macroscopic properties. It incorporates...
. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner
Lise Meitner
Lise Meitner FRS was an Austrian-born, later Swedish, physicist who worked on radioactivity and nuclear physics. Meitner was part of the team that discovered nuclear fission, an achievement for which her colleague Otto Hahn was awarded the Nobel Prize...
and Pierre Auger
Pierre Victor Auger
Pierre Victor Auger was a French physicist, born in Paris. He worked in the fields of atomic physics, nuclear physics, and cosmic ray physics....
in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik
Zeitschrift für Physik
The European Physical Journal is a joint publication of EDP Sciences, Springer Science+Business Media, and the Società Italiana di Fisica...
in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy
X-ray spectroscopy
X-ray spectroscopy is a gathering name for several spectroscopic techniques for characterization of materials by using x-ray excitation.-Characteristic X-ray Spectroscopy:...
data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy
Metallurgy
Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys. It is also the technology of metals: the way in which science is applied to their practical use...
, gas-phase chemistry, and throughout the microelectronics
Microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture of very small electronic components. Usually, but not always, this means micrometre-scale or smaller,. These devices are made from semiconductors...
industry.
Electron transitions and the Auger effect
The Auger effect is an electronic process at the heart of AES resulting from the inter- and intrastate transitions of electrons in an excited atom. When an atom is probed by an external mechanism, such as a photon or a beam of electrons with energies in the range of 2 keVElectronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
to 50 keV, a core state electron can be removed leaving behind a hole. As this is an unstable state, the core hole can be filled by an outer shell electron, whereby the electron moving to the lower energy level loses an amount of energy equal to the difference in orbital energies. The transition energy can be coupled to a second outer shell electron which will be emitted from the atom if the transferred energy is greater than the orbital binding energy. An emitted electron will have a kinetic energy of:

where
 ,
, 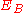 ,
, 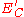 are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations. Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.
are respectively the core level, first outer shell, and second outer shell electron energies, measured from the vacuum level. The apostrophe (tic) denotes a slight modification to the binding energy of the outer shell electrons due to the ionized nature of the atom; often however, this energy modification is ignored in order to ease calculations. Since orbital energies are unique to an atom of a specific element, analysis of the ejected electrons can yield information about the chemical composition of a surface. Figure 1 illustrates two schematic views of the Auger process.The types of state-to-state transitions available to electrons during an Auger event are dependent on several factors, ranging from initial excitation energy to relative interaction rates, yet are often dominated by a few characteristic transitions. Because of the interaction between an electron's spin and orbital angular momentum (spin-orbit coupling) and the concomitant energy level splitting for various shells in an atom, there are a variety of transition pathways for filling a core hole. Energy levels are labeled using a number of different schemes such as the j-j coupling method for heavy elements (Z ≥ 75), the Russell-Saunders L-S method for lighter elements (Z < 20), and a combination of both for intermediate elements. The j-j coupling method, which is historically linked to X-ray notation
X-ray notation
X-ray notation is a method of labeling atomic orbitals that grew out of X-ray science. It is still traditionally used with most x-ray spectroscopy techniques including AES and XPS. In X-ray notation, every principal quantum number is given a letter associated with it.-Conversion:-Use:*X-ray sources...
, is almost always used to denote Auger transitions. Thus for a
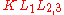 transition, K represents the core level hole,
transition, K represents the core level hole, 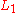 the relaxing electron's initial state, and
the relaxing electron's initial state, and 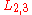 the emitted electron's initial energy state. Figure 1(b) illustrates this transition with the corresponding spectroscopic notation. The energy level of the core hole will often determine which transition types will be favored. For single energy levels, i.e. K, transitions can occur from the L levels, giving rise to strong KLL type peaks in an Auger spectrum. Higher level transitions can also occur, but are less probable. For multi-level shells, transitions are available from higher energy orbitals (different n, ℓ quantum numbers) or energy levels within the same shell (same n, different ℓ number). The result are transitions of the type LMM and KLL along with faster Coster–Kronig transitions such as LLM. while Coster–Kronig transitions are faster, they are also less energetic and thus harder to locate on an Auger spectrum. As the atomic number
the emitted electron's initial energy state. Figure 1(b) illustrates this transition with the corresponding spectroscopic notation. The energy level of the core hole will often determine which transition types will be favored. For single energy levels, i.e. K, transitions can occur from the L levels, giving rise to strong KLL type peaks in an Auger spectrum. Higher level transitions can also occur, but are less probable. For multi-level shells, transitions are available from higher energy orbitals (different n, ℓ quantum numbers) or energy levels within the same shell (same n, different ℓ number). The result are transitions of the type LMM and KLL along with faster Coster–Kronig transitions such as LLM. while Coster–Kronig transitions are faster, they are also less energetic and thus harder to locate on an Auger spectrum. As the atomic numberAtomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
Z increases, so too does the number of potential Auger transitions. Fortunately, the strongest electron-electron interactions are between levels which are close together, giving rise to characteristic peaks in an Auger spectrum. KLL and LMM peaks are some of the most commonly identified transitions during surface analysis. Finally, valence band electrons can also fill core holes or be emitted during KVV-type transitions.
Several models, both phenomenological and analytical, have been developed to describe the energetics of Auger transitions. One of the most tractable descriptions, put forth by Jenkins and Chung, estimates the energy of Auger transition ABC as:
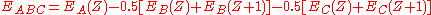
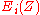 are the binding energies of the
are the binding energies of the 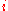 th level in element of atomic number Z and
th level in element of atomic number Z and 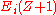 are the energies of the same levels in the next element up in the periodic table. While useful in practice, a more rigorous model accounting for effects such as screening and relaxation probabilities between energy levels gives the Auger energy as:
are the energies of the same levels in the next element up in the periodic table. While useful in practice, a more rigorous model accounting for effects such as screening and relaxation probabilities between energy levels gives the Auger energy as: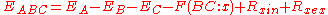
where
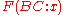 is the energy of interaction between the B and C level holes in a final atomic state x and the Rs represent intra- and extra-atomic transition energies accounting for electronic screening. Auger electron energies can be calculated based on measured values of the various
is the energy of interaction between the B and C level holes in a final atomic state x and the Rs represent intra- and extra-atomic transition energies accounting for electronic screening. Auger electron energies can be calculated based on measured values of the various 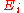 and compared to peaks in the secondary electron spectrum in order to identify chemical species. This technique has been used to compile several reference databases used for analysis in current AES setups.
and compared to peaks in the secondary electron spectrum in order to identify chemical species. This technique has been used to compile several reference databases used for analysis in current AES setups.Instrumentation
Surface sensitivity in AES arises from the fact that emitted electrons usually have energies ranging from 50 eV to 3 keV and at these values, electrons have a short mean free pathMean free path
In physics, the mean free path is the average distance covered by a moving particle between successive impacts which modify its direction or energy or other particle properties.-Derivation:...
in a solid. The escape depth of electrons is therefore localized to within a few nanometers of the target surface, giving AES an extreme sensitivity to surface species. Because of the low energy of Auger electrons, most AES setups are run under ultra-high vacuum (UHV) conditions. Such measures prevent electron scattering off of residual gas atoms as well as the formation of a thin "gas (adsorbate) layer" on the surface of the specimen which degrades analytical performance. A typical AES setup is shown schematically in figure 2. In this configuration, focused electrons are incident on a sample and emitted electrons are deflected into a cylindrical mirror analyzer (CMA). In the detection unit, Auger electrons are multiplied and the signal sent to data processing electronics. Collected Auger electrons are plotted as a function of energy against the broad secondary electron background spectrum.
Since the intensity of the Auger peaks may be small compared to the noise level of the background, AES is often run in a derivative mode which serves to highlight the peaks by modulating the electron collection current via a small applied AC voltage. Since this
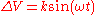 , the collection current becomes
, the collection current becomes 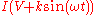 . Taylor expanding
. Taylor expandingTaylor series
In mathematics, a Taylor series is a representation of a function as an infinite sum of terms that are calculated from the values of the function's derivatives at a single point....
gives:
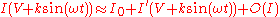
Using the setup in figure 2, detecting the signal at frequency ω will give a value for
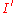 or
or  . Plotting in derivative mode also emphasizes Auger fine structure which appear as small secondary peaks surrounding the primary Auger peak. These secondary peaks, not to be confused with high energy satellites which are discussed later, arise from the presence of the same element in multiple different chemical states on a surface (i.e. Adsorbate layers) or from relaxation transitions involving valence band electrons of the substrate. Figure 3 illustrates a derivative spectrum from a copper nitride film clearly showing the Auger peaks. The peak in derivative mode is not the true Auger peak, but rather the point of maximum slope of N(E), but this concern is usually ignored.
. Plotting in derivative mode also emphasizes Auger fine structure which appear as small secondary peaks surrounding the primary Auger peak. These secondary peaks, not to be confused with high energy satellites which are discussed later, arise from the presence of the same element in multiple different chemical states on a surface (i.e. Adsorbate layers) or from relaxation transitions involving valence band electrons of the substrate. Figure 3 illustrates a derivative spectrum from a copper nitride film clearly showing the Auger peaks. The peak in derivative mode is not the true Auger peak, but rather the point of maximum slope of N(E), but this concern is usually ignored.Quantitative analysis
Semi-quantitative compositional and element analysis of a sample using AES is dependent on measuring the yield of Auger electrons during a probing event. Electron yield, in turn, depends on several critical parameters such as electron-impact cross-section and fluorescence yield. Since the Auger effect is not the only mechanism available for atomic relaxation, there is a competition between radiative and non-radiative decay processes to be the primary de-excitation pathway. The total transition rate, ω, is a sum of the non-radiative (Auger) and radiative (photon emission) processes. The Auger yield,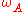 , is thus related to the fluorescence
, is thus related to the fluorescenceFluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
(x-ray) yield,
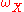 , by the relation,
, by the relation,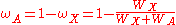
where
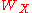 is the X-ray transition probability and
is the X-ray transition probability and 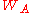 is the Auger transition probability. Attempts to relate the fluorescence and Auger yields to atomic number have resulted in plots similar to figure 4. A clear transition from electron to photon emission is evident in this chart for increasing atomic number. For heavier elements, x-ray yield becomes greater than Auger yield, indicating an increased difficulty in measuring the Auger peaks for large Z-values. Conversely, AES is sensitive to the lighter elements, and unlike X-ray fluorescence
is the Auger transition probability. Attempts to relate the fluorescence and Auger yields to atomic number have resulted in plots similar to figure 4. A clear transition from electron to photon emission is evident in this chart for increasing atomic number. For heavier elements, x-ray yield becomes greater than Auger yield, indicating an increased difficulty in measuring the Auger peaks for large Z-values. Conversely, AES is sensitive to the lighter elements, and unlike X-ray fluorescenceX-ray fluorescence
X-ray fluorescence is the emission of characteristic "secondary" X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays...
, Auger peaks can be detected for elements as light as lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
(Z = 3). Lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
represents the lower limit for AES sensitivity since the Auger effect is a "three state" event necessitating at least three electrons. Neither H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
nor He
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
can be detected with this technique. For K-level based transitions, Auger effects are dominant for Z < 15 while for L- and M-level transitions, AES data can be measured for Z ≤ 50. The yield limits effectively prescribe a cutoff for AES sensitivity, but complex techniques can be utilized to identify heavier elements, such as uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
, using the Auger effect.
Another critical quantity that determines yield of Auger electrons at a detector is the electron impact cross-section. Early approximations (in cm2) of the cross-section were based on the work of Worthington and Tomlin,
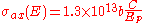
with b acting as a scaling factor between 0.25 and 0.35, and C a function of the primary electron beam energy,
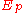 . While this value of
. While this value of 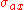 is calculated for an isolated atom, a simple modification can be made to account for matrix effects:
is calculated for an isolated atom, a simple modification can be made to account for matrix effects:
where α is the angle to the surface normal of the incident electron beam; rm can be established empirically and encompasses electron interactions with the matrix such as ionization due to backscattered electrons. Thus the total yield can be written as:
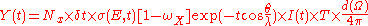
Here Nx is the number of x atoms per volume, λ the electron escape depth, θ the analyzer angle, T the transmission of the analyzer, I(t) the electron excitation flux at depth t, dΩ the solid angle, and δt is the thickness of the layer being probed. Encompassed in these terms, especially the Auger yield which is related to the transition probability, is the quantum mechanical overlap of the initial and final state wave functions. Precise expressions for the transition probability, based on first-order perturbation Hamiltonian
Hamiltonian (quantum mechanics)
In quantum mechanics, the Hamiltonian H, also Ȟ or Ĥ, is the operator corresponding to the total energy of the system. Its spectrum is the set of possible outcomes when one measures the total energy of a system...
s, can be found in Thompson and Baker. Often, all of these terms are not known, so most analyses compare measured yields with external standards of known composition. Ratios of the acquired data to standards can eliminate common terms, especially experimental setup characteristics and material parameters, and can be used to determine element composition. Comparison techniques work best for samples of homogeneous binary materials or uniform surface layers, while elemental identification is best obtained from comparison of pure samples.
Uses and limitations
There are a number of electron microscopes that have been specifically designed for use in Auger spectroscopy; these are termed scanning Auger microscopes (SAM) and can produce high resolution, spatially resolved chemical images. SAM images are obtained by stepping a focused electron beam across a sample surface and measuring the intensity of the Auger peak above the background of scattered electrons. The intensity map is correlated to a gray scale on a monitor with whiter areas corresponding to higher element concentration. In addition, sputteringSputtering
Sputtering is a process whereby atoms are ejected from a solid target material due to bombardment of the target by energetic particles. It is commonly used for thin-film deposition, etching and analytical techniques .-Physics of sputtering:...
is sometimes used with Auger spectroscopy to perform depth profiling experiments. Sputtering removes thin outer layers of a surface so that AES can be used to determine the underlying composition. Depth profiles are shown as either Auger peak height vs. sputter time or atomic concentration vs. depth. Precise depth milling through sputtering has made profiling an invaluable technique for chemical analysis of nanostructured materials and thin films. AES is also used extensively as an evaluation tool on and off fab lines in the microelectronics industry, while the versatility and sensitivity of the Auger process makes it a standard analytical tool in research labs.
Despite the advantages of high spatial resolution and precise chemical sensitivity attributed to AES, there are several factors that can limit the applicability of this technique, especially when evaluating solid specimens. One of the most common limitations encountered with Auger spectroscopy are charging effects in non-conducting samples. Charging results when the number of secondary electrons leaving the sample is different to the number of incident electrons, giving rise to a net polarity at the surface. Both positive and negative surface charges severely alter the yield of electrons emitted from the sample and hence distort the measured Auger peaks. To complicate matters, neutralization methods employed in other surface analysis techniques, such as secondary ion mass spectrometry
Secondary ion mass spectrometry
Secondary ion mass spectrometry is a technique used in materials science and surface science to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions...
(SIMS), are not applicable to AES, as these methods usually involve surface bombardment with either electrons or ions (i.e. flood gun
Flood gun
A flood gun is an electromechanical device that provides a steady flow of low-energy electrons to a desired target or "flood area."Typically, the target is an area on an insulator or semiconductor where another "writing gun" has just left a net positive charge.If the energy of a flood gun's...
). Several processes have been developed to combat the issue of charging, though none of them is ideal and still make quantification of AES data difficult. One such technique involves depositing conductive pads near the analysis area to minimize regional charging. However, this type of approach limits SAM applications as well as the amount of sample material available for probing. A related technique involves thinning or "dimpling" a non-conductive layer with Ar+
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
ions and then mounting the sample to a conductive backing prior to AES. This method has been debated, with claims that the thinning process leaves elemental artifacts on a surface and/or creates damaged layers that distort bonding and promote chemical mixing in the sample. As a result, the compositional AES data is considered suspect. The most common setup to minimize charging effects includes use of a glancing angle (~10°) electron beam and a carefully tuned bombarding energy (between 1.5 keV and 3 keV). Control of both the angle and energy can subtly alter the number of emitted electrons vis-à-vis the incident electrons and thereby reduce or altogether eliminate sample charging.
In addition to charging effects, AES data can be obscured by the presence of characteristic energy losses in a sample and higher order atomic ionization events. Electrons ejected from a solid will generally undergo multiple scattering events and lose energy in the form of collective electron density oscillations called plasmons. If plasmon losses have energies near that of an Auger peak, the less intense Auger process may become dwarfed by the plasmon peak. As Auger spectra are normally weak and spread over many eV of energy, they are difficult to extract from the background and in the presence of plasmon losses, deconvolution of the two peaks becomes extremely difficult. For such spectra, additional analysis through chemical sensitive surface techniques like x-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
(XPS) is often required to disentangle the peaks. Sometimes an Auger spectrum can also exhibit "satellite" peaks at well-defined off-set energies from the parent peak. Origin of the satellites is usually attributed to multiple ionization events in an atom or ionization cascades in which a series of electrons is emitted as relaxation occurs for core holes of multiple levels. The presence of satellites can distort the true Auger peak and/or small peak shift information due to chemical bonding at the surface. Several studies have been undertaken to further quantify satellite peaks.
Despite these sometimes substantial drawbacks, Auger electron spectroscopy is a widely used surface analysis technique that has been successfully applied to many diverse fields ranging from gas phase chemistry to nanostructure characterization. Very new class of high-resolving electrostatic energy analyzers recently developed – the face-field analyzers (FFA) can be used for remote electron spectroscopy of distant surfaces or surfaces with large roughness or even with deep dimples. These instruments are designed as if to be specifically used in combined scanning electron microscope
Scanning electron microscope
A scanning electron microscope is a type of electron microscope that images a sample by scanning it with a high-energy beam of electrons in a raster scan pattern...
s (SEMs). "FFA" in principle have no perceptible end-fields, which usually distort focusing in most of analysers known, for example, well known CMA.
Sensitivity, quantitative detail, and ease of use have brought AES from an obscure nuisance effect to a functional and practical characterization technique in just over fifty years. With applications both in the research laboratory and industrial settings, AES will continue to be a cornerstone of surface-sensitive electron-based spectroscopies.
See also
- List of materials analysis methods
- Arc mappingArc mappingArc mapping is a technique used in fire investigation and is usually performed by a forensic electrical engineer. It relies on finding the locations of electrical arcs and other electrical faults that occurred during the fire; the locations of the electrical faults can then, under some...
- Ultra-violet photoelectron spectroscopy (UPS, for gases)
- Photoemission spectroscopyPhotoemission spectroscopyPhotoemission spectroscopy , also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in a substance...
(PES, for solid surfaces) - Rydberg Ionization SpectroscopyRydberg Ionization Spectroscopy-Rydberg states:Atoms and molecules have ionization energy thresholds associated with the energy required to remove an electron from the ionic core. Rydberg series describe the energy levels associated with almost removing an electron from the ionic core...
- X-ray photoelectron spectroscopyX-ray photoelectron spectroscopyX-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...

