
X-ray fluorescence
Encyclopedia

Elemental analysis
Percent Composition is a process where a sample of some material is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualitative , and it can be quantitative...
and chemical analysis
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
, particularly in the investigation of metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s, glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
, ceramic
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
s and building materials, and for research in geochemistry
Geochemistry
The field of geochemistry involves study of the chemical composition of the Earth and other planets, chemical processes and reactions that govern the composition of rocks, water, and soils, and the cycles of matter and energy that transport the Earth's chemical components in time and space, and...
, forensic science and archaeology
Archaeology
Archaeology, or archeology , is the study of human society, primarily through the recovery and analysis of the material culture and environmental data that they have left behind, which includes artifacts, architecture, biofacts and cultural landscapes...
.
Underlying physics

Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
X-rays or to gamma rays, ionization of their component atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s may take place. Ionization consists of the ejection of one or more electrons from the atom, and may occur if the atom is exposed to radiation with an energy greater than its ionization potential
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
. X-rays and gamma rays can be energetic enough to expel tightly held electrons from the inner orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
s of the atom. The removal of an electron in this way renders the electronic structure of the atom unstable, and electrons in higher orbitals "fall" into the lower orbital to fill the hole
Electron hole
An electron hole is the conceptual and mathematical opposite of an electron, useful in the study of physics, chemistry, and electrical engineering. The concept describes the lack of an electron at a position where one could exist in an atom or atomic lattice...
left behind. In falling, energy is released in the form of a photon, the energy of which is equal to the energy difference of the two orbitals involved. Thus, the material emits radiation, which has energy characteristic of the atoms present. The term fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
is applied to phenomena in which the absorption of radiation of a specific energy results in the re-emission of radiation of a different energy (generally lower).


Characteristic radiation
Each element has electronic orbitals of characteristic energy. Following removal of an inner electron by an energetic photon provided by a primary radiation source, an electron from an outer shell drops into its place. There are a limited number of ways in which this can happen, as shown in figure 1. The main transitions are given names: an L→K transition is traditionally called Kα, an M→K transition is called Kβ, an M→L transition is called Lα, and so on. Each of these transitions yields a fluorescent photon with a characteristic energy equal to the difference in energy of the initial and final orbital. The wavelength of this fluorescent radiation can be calculated from Planck's LawPlanck postulate
The Planck Postulate , one of the fundamental principles of quantum mechanics, is the postulate that the energy of oscillators in a black body is quantized, and is given byE=nh\nu\,,...
:

The fluorescent radiation can be analysed either by sorting the energies of the photons (energy-dispersive analysis) or by separating the wavelengths of the radiation (wavelength-dispersive analysis). Once sorted, the intensity of each characteristic radiation is directly related to the amount of each element in the material. This is the basis of a powerful technique in analytical chemistry
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
. Figure 2 shows the typical form of the sharp fluorescent spectral lines obtained in the wavelength-dispersive method (see Moseley's law
Moseley's law
Moseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms. The law was discovered and published by the English physicist Henry Moseley in 1913...
).
Primary radiation
In order to excite the atoms, a source of radiation is required, with sufficient energy to expel tightly held inner electrons. Conventional X-ray generators are most commonly used, because their output can readily be "tuned" for the application, and because higher power can be deployed relative to other techniques. However, gamma ray sources can be used without the need for an elaborate power supply, allowing an easier use in small portable instruments. When the energy source is a synchrotronSynchrotron
A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant...
or the X-rays are focused by an optic like a polycapillary, the X-ray beam can be very small and very intense. As a result, atomic information on the sub-micrometre scale can be obtained. X-ray generators in the range 20–60 kV are used, which allow excitation of a broad range of atoms. The continuous spectrum consists of "bremsstrahlung
Bremsstrahlung
Bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
" radiation: radiation produced when high-energy electrons passing through the tube are progressively decelerated by the material of the tube anode (the "target"). A typical tube output spectrum is shown in figure 3.
Dispersion
In energy dispersive analysis, the fluorescent X-rays emitted by the material sample are directed into a solid-state detector which produces a "continuous" distribution of pulses, the voltages of which are proportional to the incoming photon energies. This signal is processed by a multichannel analyser (MCA) which produces an accumulating digital spectrum that can be processed to obtain analytical data. In wavelength dispersive analysis, the fluorescent X-rays emitted by the material sample are directed into a diffraction grating monochromator. The diffraction grating used is usually a single crystal. By varying the angle of incidence and take-off on the crystal, a single X-ray wavelength can be selected. The wavelength obtained is given by the Bragg EquationBragg's law
In physics, Bragg's law gives the angles for coherent and incoherent scattering from a crystal lattice. When X-rays are incident on an atom, they make the electronic cloud move as does any electromagnetic wave...
:
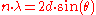
where d is the spacing of atomic layers parallel to the crystal surface.
Detection
In energy dispersive analysis, dispersion and detection are a single operation, as already mentioned above. Proportional counterProportional counter
A proportional counter is a measurement device to count particles of ionizing radiation and measure their energy.A proportional counter is a type of gaseous ionization detector. Its operation is similar to that of a Geiger-Müller counter, but uses a lower operating voltage. An inert gas is used to...
s or various types of solid-state detectors (PIN diode
PIN diode
A PIN diode is a diode with a wide, lightly doped 'near' intrinsic semiconductor region between a p-type semiconductor and an n-type semiconductor region. The p-type and n-type regions are typically heavily doped because they are used for ohmic contacts....
, Si(Li), Ge(Li), Silicon Drift Detector
Silicon drift detector
Silicon drift detectors are X-ray radiation detectors used in x-ray spectrometry and electron microscopy . Their chief characteristics compared with other X-ray detectors are:*high count rates*comparatively high energy resolution Silicon drift detectors (SDDs) are X-ray radiation detectors used...
SDD) are used. They all share the same detection principle: An incoming X-ray photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
ionises a large number of detector atoms with the amount of charge produced being proportional to the energy of the incoming photon. The charge is then collected and the process repeats itself for the next photon. Detector speed is obviously critical, as all charge carriers measured have to come from the same photon to measure the photon energy correctly (peak length discrimination is used to eliminate events that seem to have been produced by two X-ray photons arriving almost simultaneously). The spectrum is then built up by dividing the energy spectrum into discrete bins and counting the number of pulses registered within each energy bin. EDXRF
Wavelength dispersive X-ray spectroscopy
The Wavelength dispersive X-ray spectroscopy is a method used to count the number of X-rays of a specific wavelength diffracted by a crystal. The wavelength of the impinging x-ray and the crystal's lattice spacings are related by Bragg's law and produce constructive interference if they fit the...
detector types vary in resolution, speed and the means of cooling (a low number of free charge carriers is critical in the solid state detectors): proportional counters with resolutions of several hundred eV cover the low end of the performance spectrum, followed by PIN diode
PIN diode
A PIN diode is a diode with a wide, lightly doped 'near' intrinsic semiconductor region between a p-type semiconductor and an n-type semiconductor region. The p-type and n-type regions are typically heavily doped because they are used for ohmic contacts....
detectors, while the Si(Li), Ge(Li) and Silicon Drift Detectors (SDD) occupy the high end of the performance scale.
In wavelength dispersive analysis, the single-wavelength radiation produced by the monochromator is passed into a photomultiplier
Photomultiplier
Photomultiplier tubes , members of the class of vacuum tubes, and more specifically phototubes, are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum...
, a detector similar to a Geiger counter
Geiger counter
A Geiger counter, also called a Geiger–Müller counter, is a type of particle detector that measures ionizing radiation. They detect the emission of nuclear radiation: alpha particles, beta particles or gamma rays. A Geiger counter detects radiation by ionization produced in a low-pressure gas in a...
, which counts individual photons as they pass through. The counter is a chamber containing a gas that is ionised by X-ray photons. A central electrode is charged at (typically) +1700 V with respect to the conducting chamber walls, and each photon triggers a pulse-like cascade of current across this field. The signal is amplified and transformed into an accumulating digital count. These counts are then processed to obtain analytical data.
X-ray intensity
The fluorescence process is inefficient, and the secondary radiation is much weaker than the primary beam. Furthermore, the secondary radiation from lighter elements is of relatively low energy (long wavelength) and has low penetrating power, and is severely attenuated if the beam passes through air for any distance. Because of this, for high-performance analysis, the path from tube to sample to detector is maintained under high vacuum (around 10 Pa residual pressure). This means in practice that most of the working parts of the instrument have to be located in a large vacuum chamber. The problems of maintaining moving parts in vacuum, and of rapidly introducing and withdrawing the sample without losing vacuum, pose major challenges for the design of the instrument. For less demanding applications, or when the sample is damaged by a vacuum (e.g. a volatile sample), a helium-swept X-ray chamber can be substituted, with some loss of low-Z (Z = atomic numberAtomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
) intensities.
Chemical analysis
The use of a primary X-ray beam to excite fluorescent radiation from the sample was first proposed by Glocker and Schreiber in 1928. Today, the method is used as a non-destructive analytical technique, and as a process control tool in many extractive and processing industries. In principle, the lightest element that can be analysed is berylliumBeryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
(Z = 4), but due to instrumental limitations and low X-ray yields for the light elements, it is often difficult to quantify elements lighter than sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
(Z = 11), unless background corrections and very comprehensive inter-element corrections are made.

Energy dispersive spectrometry
In energy dispersive spectrometers (EDX or EDS), the detector allows the determination of the energy of the photon when it is detected. Detectors historically have been based on silicon semiconductors, in the form of lithium-drifted silicon crystals, or high-purity silicon wafers.
Si(Li) detectors
These consist essentially of a 3–5 mm thick silicon junction type p-i-n diode (same as PIN diode) with a bias of −1000 V across it. The lithium-drifted centre part forms the non-conducting i-layer, where Li compensates the residual acceptors which would otherwise make the layer p-type. When an X-ray photon passes through, it causes a swarm of electron-hole pairs to form, and this causes a voltage pulse. To obtain sufficiently low conductivity, the detector must be maintained at low temperature, and liquid-nitrogen must be used for the best resolution. With some loss of resolution, the much more convenient Peltier cooling can be employed.Wafer detectors
More recently, high-purity silicon wafers with low conductivity have become routinely available. Cooled by the Peltier effect, this provides a cheap and convenient detector, although the liquid nitrogenLiquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
cooled Si(Li) detector still has the best resolution (i.e. ability to distinguish different photon energies).
Amplifiers
The pulses generated by the detector are processed by pulse-shaping amplifiers. It takes time for the amplifier to shape the pulse for optimum resolution, and there is therefore a trade-off between resolution and count-rate: long processing time for good resolution results in "pulse pile-up" in which the pulses from successive photons overlap. Multi-photon events are, however, typically more drawn out in time (photons did not arrive exactly at the same time) than single photon events and pulse-length discrimination can thus be used to filter most of these out. Even so, a small number of pile-up peaks will remain and pile-up correction should be built into the software in applications that require trace analysis. To make the most efficient use of the detector, the tube current should be reduced to keep multi-photon events (before discrimination) at a reasonable level, e.g. 5–20%.Processing
Considerable computer power is dedicated to correcting for pulse-pile up and for extraction of data from poorly resolved spectra. These elaborate correction processes tend to be based on empirical relationships that may change with time, so that continuous vigilance is required in order to obtain chemical data of adequate precision.Usage
EDXEnergy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectroscopy is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on the investigation of an interaction of a some source of X-ray excitation and a sample...
spectrometers are superior to WDX
Wavelength dispersive X-ray spectroscopy
The Wavelength dispersive X-ray spectroscopy is a method used to count the number of X-rays of a specific wavelength diffracted by a crystal. The wavelength of the impinging x-ray and the crystal's lattice spacings are related by Bragg's law and produce constructive interference if they fit the...
spectrometers in that they are smaller, simpler in design and have fewer engineered parts. They can also use miniature X-ray tubes or gamma sources. This makes them cheaper and allows miniaturization and portability. This type of instrument is commonly used for portable quality control screening applications, such as testing toys for Lead (Pb) content, sorting scrap metals, and measuring the lead content of residential paint. On the other hand, the low resolution and problems with low count rate and long dead-time makes them inferior for high-precision analysis. They are, however, very effective for high-speed, multi-elemental analysis. Field Portable XRF analysers currently on the market weigh less than 2 kg, and have limits of detection on the order of 2 parts per million of Lead (Pb) in pure sand.
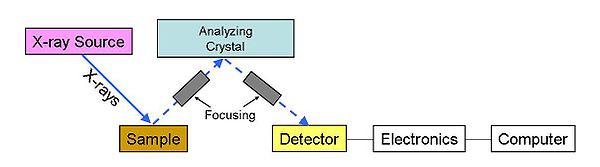

Wavelength dispersive spectrometry
In wavelength dispersiveWavelength dispersive X-ray spectroscopy
The Wavelength dispersive X-ray spectroscopy is a method used to count the number of X-rays of a specific wavelength diffracted by a crystal. The wavelength of the impinging x-ray and the crystal's lattice spacings are related by Bragg's law and produce constructive interference if they fit the...
spectrometers (WDX or WDS
Wavelength dispersive X-ray spectroscopy
The Wavelength dispersive X-ray spectroscopy is a method used to count the number of X-rays of a specific wavelength diffracted by a crystal. The wavelength of the impinging x-ray and the crystal's lattice spacings are related by Bragg's law and produce constructive interference if they fit the...
), the photons are separated by diffraction
Diffraction
Diffraction refers to various phenomena which occur when a wave encounters an obstacle. Italian scientist Francesco Maria Grimaldi coined the word "diffraction" and was the first to record accurate observations of the phenomenon in 1665...
on a single crystal before being detected. Although wavelength dispersive spectrometers are occasionally used to scan a wide range of wavelengths, producing a spectrum plot as in EDS, they are usually set up to make measurements only at the wavelength of the emission lines of the elements of interest. This is achieved in two different ways:
- "Simultaneous" spectrometers have a number of "channels" dedicated to analysis of a single element, each consisting of a fixed-geometry crystal monochromator, a detector, and processing electronics. This allows a number of elements to be measured simultaneously, and in the case of high-powered instruments, complete high-precision analyses can be obtained in under 30 s. Another advantage of this arrangement is that the fixed-geometry monochromators have no continuously moving parts, and so are very reliable. Reliability is important in production environments where instruments are expected to work without interruption for months at a time. Disadvantages of simultaneous spectrometers include relatively high cost for complex analyses, since each channel used is expensive. The number of elements that can be measured is limited to 15–20, because of space limitations on the number of monochromators that can be crowded around the fluorescing sample. The need to accommodate multiple monochromators means that a rather open arrangement around the sample is required, leading to relatively long tube-sample-crystal distances, which leads to lower detected intensities and more scattering. The instrument is inflexible, because if a new element is to be measured, a new measurement channel has to be bought and installed.
- "Sequential" spectrometers have a single variable-geometry monochromator (but usually with an arrangement for selecting from a choice of crystals), a single detector assembly (but usually with more than one detector arranged in tandem), and a single electronic pack. The instrument is programmed to move through a sequence of wavelengths, in each case selecting the appropriate X-ray tube power, the appropriate crystal, and the appropriate detector arrangement. The length of the measurement program is essentially unlimited, so this arrangement is very flexible. Because there is only one monochromator, the tube-sample-crystal distances can be kept very short, resulting in minimal loss of detected intensity. The obvious disadvantage is relatively long analysis time, particularly when many elements are being analysed, not only because the elements are measured in sequence, but also because a certain amount of time is taken in readjusting the monochromator geometry between measurements. Furthermore, the frenzied activity of the monochromator during an analysis program is a challenge for mechanical reliability. However, modern sequential instruments can achieve reliability almost as good as that of simultaneous instruments, even in continuous-usage applications.
Sample presentation
In order to keep the geometry of the tube-sample-detector assembly constant, the sample is normally prepared as a flat disc, typically of diameter 20–50 mm. This is located at a standardized, small distance from the tube window. Because the X-ray intensity follows an inverse-square law, the tolerances for this placement and for the flatness of the surface must be very tight in order to maintain a repeatable X-ray flux. Ways of obtaining sample discs vary: metals may be machined to shape, minerals may be finely ground and pressed into a tablet, and glasses may be cast to the required shape. A further reason for obtaining a flat and representative sample surface is that the secondary X-rays from lighter elements often only emit from the top few micrometres of the sample. In order to further reduce the effect of surface irregularities, the sample is usually spun at 5–20 rpm. It is necessary to ensure that the sample is sufficiently thick to absorb the entire primary beam. For higher-Z materials, a few millimetres thickness is adequate, but for a light-element matrix such as coal, a thickness of 30–40 mm is needed.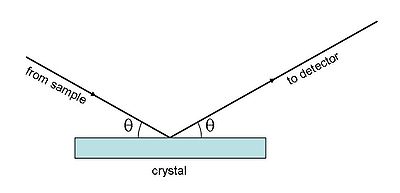
Monochromators
The common feature of monochromators is the maintenance of a symmetrical geometry between the sample, the crystal and the detector. In this geometry the Bragg diffraction condition is obtained.The X-ray emission lines are very narrow (see figure 2), so the angles must be defined with considerable precision. This is achieved in two ways:
- Flat crystal with Soller collimators
The Soller collimator is a stack of parallel metal plates, spaced a few tenths of a millimetre apart. To improve angle resolution, one must lengthen the collimator, and/or reduce the plate spacing. This arrangement has the advantage of simplicity and relatively low cost, but the collimators reduce intensity and increase scattering, and reduce the area of sample and crystal that can be "seen". The simplicity of the geometry is especially useful for variable-geometry monochromators.
- Curved crystal with slits
The Rowland circle geometry ensures that the slits are both in focus, but in order for the Bragg condition to be met at all points, the crystal must first be bent to a radius of 2R (where R is the radius of the Rowland circle), then ground to a radius of R. This arrangement allows higher intensities (typically 8-fold) with higher resolution (typically 4-fold) and lower background. However, the mechanics of keeping Rowland circle geometry in a variable-angle monochromator is extremely difficult. In the case of fixed-angle monochromators (for use in simultaneous spectrometers), crystals bent to a logarithmic spiral shape give the best focusing performance. The manufacture of curved crystals to acceptable tolerances increases their price considerably.
Analysis Lines
The spectral lines used for chemical analysis are selected on the basis of intensity, accessibility by the instrument, and lack of line overlaps. Typical lines used, and their wavelengths, are as follows:| element | line | wavelength (nm) | element | line | wavelength (nm) | element | line | wavelength (nm) | element | line | wavelength (nm) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | Kα | 22.8 | Ni | Kα1 | 0.1658 | I | Lα1 | 0.3149 | Pt | Lα1 | 0.1313 | |||
| Be | Kα | 11.4 | Cu | Kα1 | 0.1541 | Xe | Lα1 | 0.3016 | Au | Lα1 | 0.1276 | |||
| B | Kα | 6.76 | Zn | Kα1 | 0.1435 | Cs | Lα1 | 0.2892 | Hg | Lα1 | 0.1241 | |||
| C | Kα | 4.47 | Ga | Kα1 | 0.1340 | Ba | Lα1 | 0.2776 | Tl | Lα1 | 0.1207 | |||
| N | Kα | 3.16 | Ge | Kα1 | 0.1254 | La | Lα1 | 0.2666 | Pb | Lα1 | 0.1175 | |||
| O | Kα | 2.362 | As | Kα1 | 0.1176 | Ce | Lα1 | 0.2562 | Bi | Lα1 | 0.1144 | |||
| F | Kα1,2 | 1.832 | Se | Kα1 | 0.1105 | Pr | Lα1 | 0.2463 | Po | Lα1 | 0.1114 | |||
| Ne | Kα1,2 | 1.461 | Br | Kα1 | 0.1040 | Nd | Lα1 | 0.2370 | At | Lα1 | 0.1085 | |||
| Na | Kα1,2 | 1.191 | Kr | Kα1 | 0.09801 | Pm | Lα1 | 0.2282 | Rn | Lα1 | 0.1057 | |||
| Mg | Kα1,2 | 0.989 | Rb | Kα1 | 0.09256 | Sm | Lα1 | 0.2200 | Fr | Lα1 | 0.1031 | |||
| Al | Kα1,2 | 0.834 | Sr | Kα1 | 0.08753 | Eu | Lα1 | 0.2121 | Ra | Lα1 | 0.1005 | |||
| Si | Kα1,2 | 0.7126 | Y | Kα1 | 0.08288 | Gd | Lα1 | 0.2047 | Ac | Lα1 | 0.0980 | |||
| P | Kα1,2 | 0.6158 | Zr | Kα1 | 0.07859 | Tb | Lα1 | 0.1977 | Th | Lα1 | 0.0956 | |||
| S | Kα1,2 | 0.5373 | Nb | Kα1 | 0.07462 | Dy | Lα1 | 0.1909 | Pa | Lα1 | 0.0933 | |||
| Cl | Kα1,2 | 0.4729 | Mo | Kα1 | 0.07094 | Ho | Lα1 | 0.1845 | U | Lα1 | 0.0911 | |||
| Ar | Kα1,2 | 0.4193 | Tc | Kα1 | 0.06751 | Er | Lα1 | 0.1784 | Np | Lα1 | 0.0888 | |||
| K | Kα1,2 | 0.3742 | Ru | Kα1 | 0.06433 | Tm | Lα1 | 0.1727 | Pu | Lα1 | 0.0868 | |||
| Ca | Kα1,2 | 0.3359 | Rh | Kα1 | 0.06136 | Yb | Lα1 | 0.1672 | Am | Lα1 | 0.0847 | |||
| Sc | Kα1,2 | 0.3032 | Pd | Kα1 | 0.05859 | Lu | Lα1 | 0.1620 | Cm | Lα1 | 0.0828 | |||
| Ti | Kα1,2 | 0.2749 | Ag | Kα1 | 0.05599 | Hf | Lα1 | 0.1570 | Bk | Lα1 | 0.0809 | |||
| V | Kα1 | 0.2504 | Cd | Kα1 | 0.05357 | Ta | Lα1 | 0.1522 | Cf | Lα1 | 0.0791 | |||
| Cr | Kα1 | 0.2290 | In | Lα1 | 0.3772 | W | Lα1 | 0.1476 | Es | Lα1 | 0.0773 | |||
| Mn | Kα1 | 0.2102 | Sn | Lα1 | 0.3600 | Re | Lα1 | 0.1433 | Fm | Lα1 | 0.0756 | |||
| Fe | Kα1 | 0.1936 | Sb | Lα1 | 0.3439 | Os | Lα1 | 0.1391 | Md | Lα1 | 0.0740 | |||
| Co | Kα1 | 0.1789 | Te | Lα1 | 0.3289 | Ir | Lα1 | 0.1351 | No | Lα1 | 0.0724 | |||
Other lines are often used, depending on the type of sample and equipment available.
Crystals
The desirable characteristics of a diffraction crystal are:- High diffraction intensity
- High dispersion
- Narrow diffracted peak width
- High peak-to-background
- Absence of interfering elements
- Low thermal coefficient of expansion
- Stability in air and on exposure to X-rays
- Ready availability
- Low cost
Crystals with simple structure tend to give the best diffraction performance. Crystals containing heavy atoms can diffract well, but also fluoresce themselves, causing interference. Crystals that are water-soluble, volatile or organic tend to give poor stability.
Commonly used crystal materials include LiF (lithium fluoride), ADP (ammonium dihydrogen phosphate), Ge (germanium), graphite, InSb (indium antimonide), PE (tetrakis-(hydroxymethyl)-methane: penta-erythritol), KAP (potassium hydrogen phthalate), RbAP (rubidium hydrogen phthalate) and TlAP (thallium(I) hydrogen phthalate). In addition, there is an increasing use of "layered synthetic microstructures", which are "sandwich" structured materials comprising successive thick layers of low atomic number matrix, and monatomic layers of a heavy element. These can in principle be custom-manufactured to diffract any desired long wavelength, and are used extensively for elements in the range Li to Mg.
Properties of commonly used crystals
| material | plane | d (nm) | min λ (nm) | max λ (nm) | intensity | thermal expansion | durability |
|---|---|---|---|---|---|---|---|
| LiF | 200 | 0.2014 | 0.053 | 0.379 | |||
| LiF | 220 | 0.1424 | 0.037 | 0.268 | |||
| LiF | 420 | 0.0901 | 0.024 | 0.169 | |||
| ADP | 101 | 0.5320 | 0.139 | 1.000 | |||
| Ge | 111 | 0.3266 | 0.085 | 0.614 | |||
| graphite | 001 | 0.3354 | 0.088 | 0.630 | |||
| InSb | 111 | 0.3740 | 0.098 | 0.703 | |||
| PE | 002 | 0.4371 | 0.114 | 0.821 | |||
| KAP | 1010 | 1.325 | 0.346 | 2.490 | |||
| RbAP | 1010 | 1.305 | 0.341 | 2.453 | |||
| Si | 111 | 0.3135 | 0.082 | 0.589 | |||
| TlAP | 1010 | 1.295 | 0.338 | 2.434 | |||
| YB66 | 400 | 0.586 | |||||
| 6 nm LSM | |||||||
| 6.00 | 1.566 | 11.276 |
Detectors
Detectors used for wavelength dispersive spectrometry need to have high pulse processing speeds in order to cope with the very high photon count rates that can be obtained. In addition, they need sufficient energy resolution to allow filtering-out of background noise and spurious photons from the primary beam or from crystal fluorescence. There are four common types of detector:- gas flow proportional counters
- sealed gas detectors
- scintillation counters
- semiconductor detectors

Sealed gas detectors are similar to the gas flow proportional counter, except that the gas does not flow through it. The gas is usually krypton or xenon at a few atmospheres pressure. They are applied usually to wavelengths in the 0.15–0.6 nm range. They are applicable in principle to longer wavelengths, but are limited by the problem of manufacturing a thin window capable of withstanding the high pressure difference.
Scintillation counters consist of a scintillating crystal (typically of sodium iodide doped with thallium) attached to a photomultiplier. The crystal produces a group of scintillations for each photon absorbed, the number being proportional to the photon energy. This translates into a pulse from the photomultiplier of voltage proportional to the photon energy. The crystal must be protected with a relatively thick aluminium/beryllium foil window, which limits the use of the detector to wavelengths below 0.25 nm. Scintillation counters are often connected in series with a gas flow proportional counter: the latter is provided with an outlet window opposite the inlet, to which the scintillation counter is attached. This arrangement is particularly used in sequential spectrometers.
Semiconductor detectors can be used in theory, and their applications are increasing as their technology improves, but historically their use for WDX has been restricted by their slow response (see EDX
Energy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectroscopy is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on the investigation of an interaction of a some source of X-ray excitation and a sample...
).
Extracting analytical results
At first sight, the translation of X-ray photon count-rates into elemental concentrations would appear to be straightforward: WDX separates the X-ray lines efficiently, and the rate of generation of secondary photons is proportional to the element concentration. However, the number of photons leaving the sample is also affected by the physical properties of the sample: so-called "matrix effects". These fall broadly into three categories:- X-ray absorption
- X-ray enhancement
- sample macroscopic effects
All elements absorb X-rays to some extent. Each element has a characteristic absorption spectrum which consists of a "saw-tooth" succession of fringes, each step-change of which has wavelength close to an emission line of the element. Absorption attenuates the secondary X-rays leaving the sample. For example, the mass absorption coefficient of silicon at the wavelength of the aluminium Kα line is 50 m²/kg, whereas that of iron is 377 m²/kg. This means that a given concentration of aluminium in a matrix of iron gives only one seventh of the count rate compared with the same concentration of aluminium in a silicon matrix. Fortunately, mass absorption coefficients are well known and can be calculated. However, to calculate the absorption for a multi-element sample, the composition must be known. For analysis of an unknown sample, an iterative procedure is therefore used. It will be noted that, to derive the mass absorption accurately, data for the concentration of elements not measured by XRF may be needed, and various strategies are employed to estimate these. As an example, in cement analysis, the concentration of oxygen (which is not measured) is calculated by assuming that all other elements are present as standard oxides.
Enhancement occurs where the secondary X-rays emitted by a heavier element are sufficiently energetic to stimulate additional secondary emission from a lighter element. This phenomenon can also be modelled, and corrections can be made provided that the full matrix composition can be deduced.
Sample macroscopic effects consist of effects of inhomogeneities of the sample, and unrepresentative conditions at its surface. Samples are ideally homogeneous and isotropic, but they often deviate from this ideal. Mixtures of multiple crystalline components in mineral powders can result in absorption effects that deviate from those calculable from theory. When a powder is pressed into a tablet, the finer minerals concentrate at the surface. Spherical grains tend to migrate to the surface more than do angular grains. In machined metals, the softer components of an alloy tend to smear across the surface. Considerable care and ingenuity are required to minimize these effects. Because they are artifacts of the method of sample preparation, these effects can not be compensated by theoretical corrections, and must be "calibrated in". This means that the calibration materials and the unknowns must be compositionally and mechanically similar, and a given calibration is applicable only to a limited range of materials. Glasses most closely approach the ideal of homogeneity and isotropy, and for accurate work, minerals are usually prepared by dissolving them in a borate glass, and casting them into a flat disc or "bead". Prepared in this form, a virtually universal calibration is applicable.
Further corrections that are often employed include background correction and line overlap correction. The background signal in an XRF spectrum derives primarily from scattering of primary beam photons by the sample surface. Scattering varies with the sample mass absorption, being greatest when mean atomic number is low. When measuring trace amounts of an element, or when measuring on a variable light matrix, background correction becomes necessary. This is really only feasible on a sequential spectrometer. Line overlap is a common problem, bearing in mind that the spectrum of a complex mineral can contain several hundred measurable lines. Sometimes it can be overcome by measuring a less-intense, but overlap-free line, but in certain instances a correction is inevitable. For instance, the Kα is the only usable line for measuring sodium, and it overlaps the zinc Lβ (L2-M4) line. Thus zinc, if present, must be analysed in order to properly correct the sodium value.
Other spectroscopic methods using the same principle
It is also possible to create a characteristic secondary X-ray emission using other incident radiation to excite the sample:- electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
beam: electron microprobeElectron microprobeAn electron microprobe , also known as an electron probe microanalyzer or electron micro probe analyzer , is an analytical tool used to non-destructively determine the chemical composition of small volumes of solid materials...
(or Castaing microprobe); - ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
beam: particle induced X-ray emissionPIXEParticle-induced X-ray emission or proton-induced X-ray emission is a technique used in the determining of the elemental make-up of a material or sample. When a material is exposed to an ion beam, atomic interactions occur that give off EM radiation of wavelengths in the x-ray part of the...
(PIXE).
When radiated by an X-ray beam, the sample also emits other radiations that can be used for analysis:
- electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s ejected by the photoelectric effectPhotoelectric effectIn the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
: X-ray photoelectron spectroscopyX-ray photoelectron spectroscopyX-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
(XPS), also called electron spectroscopy for chemical analysisX-ray photoelectron spectroscopyX-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
(ESCA)
The de-excitation also ejects Auger electron
Auger electron
The Auger effect is a physical phenomenon in which the transition of an electron in an atom filling in an inner-shell vacancy causes the emission of another electron. When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in...
s, but Auger electron spectroscopy
Auger electron spectroscopy
Auger electron spectroscopy is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science...
(AES) normally uses an electron beam as the probe.
Confocal microscopy
Confocal
In geometry, confocal means having the same foci.* For an optical cavity consisting of two mirrors, confocal means that they share their foci...
X-ray fluorescence imaging is a newer technique that allow control over depth, in addition to horizontal and vertical aiming, for example, when analysing buried layers in a painting.
Instrument qualification
A 2001 review, addresses the application of portable instrumentation from QAQuality Assurance
Quality assurance, or QA for short, is the systematic monitoring and evaluation of the various aspects of a project, service or facility to maximize the probability that minimum standards of quality are being attained by the production process...
/QC
Quality control
Quality control, or QC for short, is a process by which entities review the quality of all factors involved in production. This approach places an emphasis on three aspects:...
perspectives. It provides a guide to the development of a set of SOPs if regulatory compliance guidelines are not available.
See also
- Emission spectroscopy
- List of materials analysis methods
- Micro-X-ray fluorescenceMicro-X-ray fluorescence- Micro-X-Ray Fluorescence :Micro-x-ray fluorescence is among the newest technology used to detect fingerprints. It is a new visualization technique which rapidly reveals the elemental composition of a sample by irradiating it with a thin beam of X-rays without disturbing the sample. It was...
- Mössbauer effectMössbauer effectThe Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil-free emission and absorption of γ radiation by atomic nuclei bound in a solid...
, resonant fluorescence of gamma rays - X-ray fluorescence holography

