
Structural formula
Encyclopedia
The structural formula of a chemical compound
is a graphical representation of the molecular structure, showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Also several other formats are used, as in chemical database
s, such as SMILES, InChI and CML
.
Unlike chemical formulas
or chemical names, structural formulas provide a representation of the molecular structure. Chemists nearly always describe a chemical reaction
or synthesis
using structural formulas rather than chemical names, because the structural formulas allow the chemist to visualize the molecules and the changes that occur.
Many chemical compounds exist in different isomer
ic forms, which have different structures but the same overall chemical formula
. A structural formula indicates the arrangements of atoms in a way that a chemical formula cannot.
s (or "Lewis dot structures") are flat graphical formulas that show atom connectivity and lone pair
or unpaired
electrons, but not three-dimensional structure. This notation is mostly used for small molecules. Each line represents the two electrons of a single bond. Two or three parallel lines between pairs of atoms represent double or triple bonds, respectively. Alternatively, pairs of dots may used to represent bonding pairs. In addition, all non-bonded electrons (paired or unpaired) and any formal charges on atoms are indicated.
CH3CH2OH (ethanol
)
Parentheses are used to indicate multiple identical groups, indicating attachment to the nearest non-hydrogen atom on the left when appearing within a formula, or to the atom on the right when appearing at the start of a formula:
(CH3)2CHOH or CH(CH3)2OH (2-propanol)
In all cases, all atoms are shown, including hydrogen atoms.
s are the standard notation for more complex organic molecules. First used by the organic chemist Friedrich August Kekulé von Stradonitz
the carbon atoms in this type of diagram are implied to be located at the vertices
(corners) and termini of line segments rather than being indicated with the atomic symbol C. Hydrogen atoms attached to carbon atoms are not indicated: each carbon atom is understood to be associated with enough hydrogen atoms to give the carbon atom four bonds. The presence of a positive or negative charge
at a carbon atom takes the place of one of the implied hydrogen atoms. Hydrogen atoms attached to atoms other than carbon must be written explicitly.
).
in skeletal formulas is indicated by the Natta projection
method. Solid or dashed wedged bonds represent bonds pointing above-the-plane or below-the-plane of the paper, respectively.
molecule with a wavy bond to the HOCH2- group at the left. In this case the two possible ring structures are in chemical equilibrium with each other and also with the open-chain structure. The ring continually opens and closes, sometimes closing with one stereochemistry and sometimes with the other.
and the sawhorse
projection are used to depict specific conformers or to distinguish vicinal
stereochemistry. In both cases, two specific carbon atoms and their connecting bond are the center of attention. The only difference is a slightly different perspective: the Newman projection looking straight down the bond of interest, the sawhorse projection looking at the same bond but from a somewhat oblique
vantage point. In the Newman projection, a circle is used to represent a plane perpendicular to the bond, distinguishing the substituents on the front carbon from the substituents on the back carbon. In the sawhorse projection, the front carbon is usually on the left and is always slightly lower:
and other small-ring compounds can be shown using a standard convention. For example, the standard chair conformation of cyclohexane involves a perspective view from slightly above the average plane of the carbon atoms and indicates clearly which groups are axial and which are equatorial. Bonds in front may or may not be highlighted with stronger lines or wedges.
is used for cyclic sugar
s. Axial and equatorial positions are not distinguished; instead, substituents are positioned directly above or below the ring atom to which they are connected. Hydrogen substituents are typically omitted.
is mostly used for linear monosaccharides. At any given carbon center, vertical bond lines are equivalent to stereochemical hashed markings, directed away from the observer, while horizontal lines are equivalent to wedges, pointing toward the observer. The projection is totally unrealistic, as a saccharide would never adopt this multiply eclipsed
conformation. Nonetheless, the Fischer projection is a simple way of depicting multiple sequential stereocenters that does not require or imply any knowledge of actual conformation:
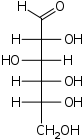
Fischer projection of D-Glucose
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
is a graphical representation of the molecular structure, showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Also several other formats are used, as in chemical database
Chemical database
A chemical database is a database specifically designed to store chemical information. This information is about chemical and crystal structures, spectra, reactions and syntheses, and thermophysical data.- Chemical structures :...
s, such as SMILES, InChI and CML
Chemical Markup Language
CML is an approach to managing molecular information using tools such as XML and Java. It was the first domain specific implementation based strictly on XML, first based on a DTD and later on XML Schema, the most robust and widely used system for precise information management in many areas...
.
Unlike chemical formulas
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
or chemical names, structural formulas provide a representation of the molecular structure. Chemists nearly always describe a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
or synthesis
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
using structural formulas rather than chemical names, because the structural formulas allow the chemist to visualize the molecules and the changes that occur.
Many chemical compounds exist in different isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
ic forms, which have different structures but the same overall chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
. A structural formula indicates the arrangements of atoms in a way that a chemical formula cannot.
Lewis structures
Lewis structureLewis structure
Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds...
s (or "Lewis dot structures") are flat graphical formulas that show atom connectivity and lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
or unpaired
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
electrons, but not three-dimensional structure. This notation is mostly used for small molecules. Each line represents the two electrons of a single bond. Two or three parallel lines between pairs of atoms represent double or triple bonds, respectively. Alternatively, pairs of dots may used to represent bonding pairs. In addition, all non-bonded electrons (paired or unpaired) and any formal charges on atoms are indicated.
Condensed formulas
In early organic-chemistry publications, where use of graphics was severely limited, a typographic system arose to describe organic structures in a line of text. Although this system tends to be problematic in application to cyclic compounds, it remains a convenient way to represent simple structures:CH3CH2OH (ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
)
Parentheses are used to indicate multiple identical groups, indicating attachment to the nearest non-hydrogen atom on the left when appearing within a formula, or to the atom on the right when appearing at the start of a formula:
(CH3)2CHOH or CH(CH3)2OH (2-propanol)
In all cases, all atoms are shown, including hydrogen atoms.
Skeletal formulas
Skeletal formulaSkeletal formula
The skeletal formula of an organic compound is a shorthand representation of its molecular structure, developed by the organic chemist, Friedrich August Kekulé von Stradonitz. Skeletal formulae are ubiquitous in organic chemistry, because they are relatively quick and simple to draw. Carbon and...
s are the standard notation for more complex organic molecules. First used by the organic chemist Friedrich August Kekulé von Stradonitz
Friedrich August Kekulé von Stradonitz
Friedrich August Kekule von Stradonitz was a German organic chemist. From the 1850s until his death, Kekule was one of the most prominent chemists in Europe, especially in theoretical chemistry...
the carbon atoms in this type of diagram are implied to be located at the vertices
Vertex (geometry)
In geometry, a vertex is a special kind of point that describes the corners or intersections of geometric shapes.-Of an angle:...
(corners) and termini of line segments rather than being indicated with the atomic symbol C. Hydrogen atoms attached to carbon atoms are not indicated: each carbon atom is understood to be associated with enough hydrogen atoms to give the carbon atom four bonds. The presence of a positive or negative charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
at a carbon atom takes the place of one of the implied hydrogen atoms. Hydrogen atoms attached to atoms other than carbon must be written explicitly.
Indication of stereochemistry
Several methods exist to picture the three-dimensional arrangement of atoms in a molecule (stereochemistryStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
).
Stereochemistry in skeletal formulas
ChiralityChirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
in skeletal formulas is indicated by the Natta projection
Natta projection
The Natta projection is a way to depict molecules with complete stereochemistry in two dimensions in a skeletal formula. This concept is named after Giulio Natta...
method. Solid or dashed wedged bonds represent bonds pointing above-the-plane or below-the-plane of the paper, respectively.
Unspecified stereochemistry
Wavy single bonds represent unknown or unspecified stereochemistry or a mixture of isomers. For example the diagram below shows the fructoseFructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
molecule with a wavy bond to the HOCH2- group at the left. In this case the two possible ring structures are in chemical equilibrium with each other and also with the open-chain structure. The ring continually opens and closes, sometimes closing with one stereochemistry and sometimes with the other.
Newman projection and sawhorse projection
The Newman projectionNewman projection
A Newman projection, useful in alkane stereochemistry, visualizes chemical conformations of a carbon-carbon chemical bond from front to back, with the front carbon represented by a dot and the back carbon as a circle . The front carbon atom is called proximal, while the back atom is called distal...
and the sawhorse
Sawhorse
A sawhorse is a beam with four legs used to support a board or plank for sawing. A pair of sawhorses can support a plank, forming a scaffold. In certain circles, it is also known as a mule.The sawhorse may be designed to fold for storage...
projection are used to depict specific conformers or to distinguish vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
stereochemistry. In both cases, two specific carbon atoms and their connecting bond are the center of attention. The only difference is a slightly different perspective: the Newman projection looking straight down the bond of interest, the sawhorse projection looking at the same bond but from a somewhat oblique
Oblique projection
Oblique projection is a simple type of graphical projection used for producing pictorial, two-dimensional images of three-dimensional objects.- Overview :Oblique projection is a type of parallel projection:...
vantage point. In the Newman projection, a circle is used to represent a plane perpendicular to the bond, distinguishing the substituents on the front carbon from the substituents on the back carbon. In the sawhorse projection, the front carbon is usually on the left and is always slightly lower:
Cyclohexane conformations
Certain conformations of cyclohexaneCyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
and other small-ring compounds can be shown using a standard convention. For example, the standard chair conformation of cyclohexane involves a perspective view from slightly above the average plane of the carbon atoms and indicates clearly which groups are axial and which are equatorial. Bonds in front may or may not be highlighted with stronger lines or wedges.
Haworth projection
The Haworth projectionHaworth projection
A Haworth projection is a common way of representing the cyclic structure of monosaccharides with a simple three-dimensional perspective.The Haworth projection was named after the English chemist Sir Norman Haworth....
is used for cyclic sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s. Axial and equatorial positions are not distinguished; instead, substituents are positioned directly above or below the ring atom to which they are connected. Hydrogen substituents are typically omitted.
Fischer projection
The Fischer projectionFischer projection
The Fischer projection, devised by Hermann Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and...
is mostly used for linear monosaccharides. At any given carbon center, vertical bond lines are equivalent to stereochemical hashed markings, directed away from the observer, while horizontal lines are equivalent to wedges, pointing toward the observer. The projection is totally unrealistic, as a saccharide would never adopt this multiply eclipsed
Eclipsed
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X-A-B-Y is 0°. Such a conformation exists in any open chain single chemical bond connecting two sp3 hybridised atoms, and is...
conformation. Nonetheless, the Fischer projection is a simple way of depicting multiple sequential stereocenters that does not require or imply any knowledge of actual conformation:

Fischer projection of D-Glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...

