
SAXS
Encyclopedia
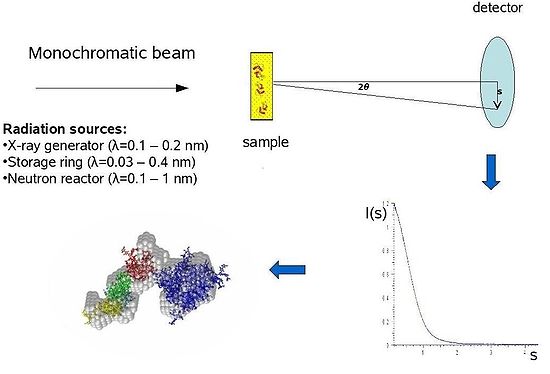
Small-angle X-ray scattering
Small-angle X-ray scattering is a small-angle scattering technique where the elastic scattering of X-rays by a sample which has inhomogeneities in the nm-range, is recorded at very low angles...
) and small-angle neutron scattering (SANS) are the two complementary techniques known jointly as small-angle scattering (SAS). SAS is an analogous method to X-ray and neutron diffraction
Neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material: A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of...
, wide angle X-ray scattering
Wide angle X-ray scattering
Wide angle X-ray scattering or Wide angle X-ray diffraction is an X-ray diffraction technique that is often used to determine the crystalline structure of polymers...
, as well as to static light scattering
Static light scattering
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight Mw of a macromolecule like a polymer or a protein. Measurement of the scattering intensity at many angles allows calculation of the root mean square...
. In separation to the other X-ray and neutron scattering methods, SAS yields information on the sizes and shapes of both crystalline and non-crystalline particles. When used to study biological materials, which are very often in aqueous solution, the scattering pattern is orientation averaged.
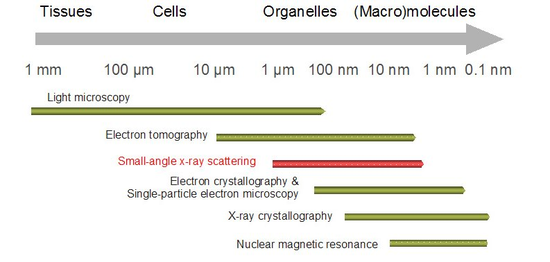
SAS patterns are collected at very small angles (a few degrees). SAS is capable of delivering structural information in the resolution range between 1 and 25 nm, and of repeat distances in partially ordered systems of up to 150 nm in size. Ultra small-angle scattering (USAS) can resolve even larger dimensions. The grazing-incidence small-angle scattering (GISAS) is a powerful technique for studying of biological molecule layers on surfaces.
In biological applications SAS is used to determine the structure of a particle in terms of average particle size and shape. One can also get information on the surface
Surface
In mathematics, specifically in topology, a surface is a two-dimensional topological manifold. The most familiar examples are those that arise as the boundaries of solid objects in ordinary three-dimensional Euclidean space R3 — for example, the surface of a ball...
-to-volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
ratio. Typically, the biological macromolecule
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
s are dispersed in a liquid. The method is accurate, mostly non-destructive and usually requires only a minimum of sample preparation. Although, biological molecules are always susceptible to radiation damage
Radiation damage
Radiation damage is a term associated with ionizing radiation.-Causes:This radiation may take several forms:*Cosmic rays and subsequent energetic particles caused by their collision with the atmosphere and other materials....
.
Conceptually, small-angle scattering experiments are simple: the sample is exposed to X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s or neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s and the scattered radiation is registered by a detector. As the SAS measurements are performed very close to the primary beam ("small angles"), the technique needs a highly collimated or focused
Focus (optics)
In geometrical optics, a focus, also called an image point, is the point where light rays originating from a point on the object converge. Although the focus is conceptually a point, physically the focus has a spatial extent, called the blur circle. This non-ideal focusing may be caused by...
X-ray or neutron beam. The biological small-angle X-ray scattering is often performed at synchrotron radiation
Synchrotron radiation
The electromagnetic radiation emitted when charged particles are accelerated radially is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers...
sources, because biological molecules normally scatter weakly and the measured solutions are dilute
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
. The biological SAXS method profits from the high intensity of X-ray photon beams provided by the synchrotron storage rings
Synchrotron
A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant...
. The X-ray or neutron scattering curve (intensity
Intensity (physics)
In physics, intensity is a measure of the energy flux, averaged over the period of the wave. The word "intensity" here is not synonymous with "strength", "amplitude", or "level", as it sometimes is in colloquial speech...
versus scattering angle) is used to create a low-resolution model of a protein, shown here on the right picture. One can further use the X-ray or neutron scattering data and fit separate domains (X-ray or NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
structures) into the "SAXS envelope".
In comparison to other structure determination methods, such as NMR or X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
, SAS allows one to overcome some restraints. For example, NMR is limited to protein size, whereas SAS can be used for small molecules as well as for large multi-molecular assemblies. Structure determination by X-ray crystallography may take several weeks or even years, whereas SAS measurements take days. However, with SAS it is not possible to measure the positions of the atoms within the molecule.
Definition
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
λ typically around 0.15 nm) or thermal neutrons (λ≈0.5 nm). The scattered intensity I(s) is recorded as a function of momentum transfer s (s=4πsinθ/λ, where 2θ is the angle between the incident and scattered radiation). From the intensity of the solution the scattering from only the solvent is subtracted. The random positions and orientations of particles result in an isotropic intensity distribution which, for monodisperse
Monodisperse
A collection of objects are called monodisperse, or monosized, if they have the same size and shape when discussing particles, and the same mass when discussing polymers...
non-interacting particles, is proportional to the scattering from a single particle averaged over all orientations. The net particle scattering is proportional to the squared difference in scattering length density (electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
for X-rays and nuclear/spin density for neutrons) between particle and solvent – the so-called contrast. The contrast can be varied in neutron scattering using H2O/D2O
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
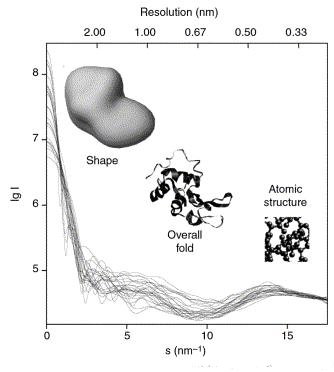
mixtures or selective deuteration to yield additional information. The information content of SAS data is illustrated here in the figure on the left, which shows X-ray scattering patterns from proteins with different folds
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
and molecular mass
Molecular mass
The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
es. At low angles (2-3 nm resolution) the curves are rapidly decaying functions of s essentially determined by the particle shape, which clearly differ. At medium resolution (2 to 0.5 nm) the differences are already less pronounced and above 0.5 nm resolution all curves are very similar. SAS thus contains information about the gross structural features – shape, quaternary and tertiary structure – but is not suitable for the analysis of the atomic structure.
History
First applications date back to the late 1930s when the main principles of SAXS were developed in the fundamental work of Guinier following his studies of metallic alloys. In the first monograph on SAXS by Guinier and Fournet it was already demonstrated that the method yields not only information on the sizes and shapes of particles but also on the internal structure of disordered and partially ordered systems.In the 1960s, the method became increasingly important in the study of biological macromolecules in solution as it allowed one to get low-resolution structural information on the overall shape and internal structure in the absence of crystals. A breakthrough in SAXS and SANS experiments came in the 1970s, thanks to the availability of synchrotron radiation
Synchrotron radiation
The electromagnetic radiation emitted when charged particles are accelerated radially is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers...
and neutron sources, the latter paving the way for contrast variation by solvent exchange of H2O for D2O and specific deuteration methods. It was realised that scattering studies on solution provide, at a minimal investment of time and effort, useful insights into the structure of non-crystalline biochemical systems. Moreover, SAXS/SANS also made possible real time investigations of intermolecular interactions, including assembly and large-scale conformational changes in macromolecular assemblies.
The main challenge of SAS as a structural method is to extract information about the three-dimensional structure of the object from the one-dimensional experimental data. In the past, only overall particle parameters (e.g. volume, radius of gyration) of the macromolecules were directly determined from the experimental data, whereas the analysis in terms of three-dimensional models was limited to simple geometrical bodies (e.g. ellipsoids, cylinders, etc.) or was performed on an ad hoc trial-and-error basis. Electron microscopy was often used as a constraint in building consensus models. In the 1980s, progress in other structural methods led to a decline of the interest of biochemists in SAS studies, which drew structural conclusions from just a couple of overall parameters or were based on trial-and-error models.
The 1990s brought a breakthrough in SAXS/SANS data analysis methods, which opened the way for reliable ab initio modelling of macromolecular complexes, including detailed determination of shape and domain structure and application of rigid body refinement techniques. This progress was accompanied by further advances in instrumentation, allowing sub-ms time resolutions to be achieved on third generation SR sources in the studies of protein and nucleic acid folding.
In 2005, a four-year project was started. Small-Angle X-Ray scattering Initiative for EuRope (SAXIER) with the goal to combine SAXS methods with other analytical techniques and create automated software to rapidly analyse large quantities of data. The project created a unified European SAXS infrastructure, using the most advanced methods available.
SAS data analysis
In a good quality SAS experiment, several solutions with varying concentrations of the macromolecule under investigation are measured. By extrapolating the scattering curves measured at different concentrations to zero concentration, one is able to obtain a scattering curve that represents infinite dilution. Then concentration effects should not affect the scattering curve. Data analysis of the extrapolated scattering curve begins with the inspection of the start of the scattering curve in the region around q = 0. If the region follows the Guinier approximation (also known as Guinier law), the sample is not aggregatedAggregation
Aggregation may refer to uses in:* Business and economics:** Aggregation problem ** Purchasing aggregation, the joining of multiple purchasers in a group purchasing organization to increase their buying power...
. Then the shape of the particle in question can be determined by various methods, of which some are described in the following reference.
Indirect Fourier transform
First step is usually to compute a Fourier transformIndirect Fourier transform
Indirect Fourier transform is a solution of ill-posed given by Fourier transform of extremely noisy data proposed by Glatter....
of the scattering curve. Transformed curve can be interpreted as distance distribution function
Radial distribution function
In statistical mechanics, a radial distribution function , g, describes how the atomic density varies as a function of the distance from one particular atom....
inside a particle. This transformation gives also a benefit of regularization
Regularization (mathematics)
In mathematics and statistics, particularly in the fields of machine learning and inverse problems, regularization involves introducing additional information in order to solve an ill-posed problem or to prevent overfitting...
of input data.
Low-resolution models
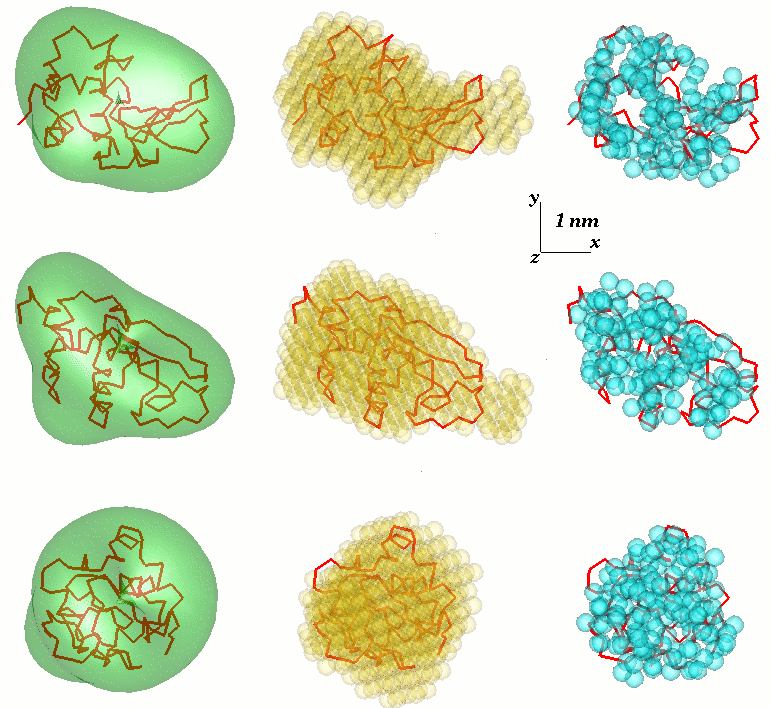
Freely available SAS analysis computer programs have been intensively developed at EMBL
European Molecular Biology Laboratory
The European Molecular Biology Laboratory is a molecular biology research institution supported by 20 European countries and Australia as associate member state. EMBL was created in 1974 and is an intergovernmental organisation funded by public research money from its member states...
. In the first general ab initio approach, an angular envelope function of the particle r=F(ω), where (r,ω) are spherical coordinates, is described by a series of spherical harmonics
Spherical harmonics
In mathematics, spherical harmonics are the angular portion of a set of solutions to Laplace's equation. Represented in a system of spherical coordinates, Laplace's spherical harmonics Y_\ell^m are a specific set of spherical harmonics that forms an orthogonal system, first introduced by Pierre...
. The low resolution shape is thus defined by a few parameters – the coefficients of this series – which fit the scattering data. The approach was further developed and implemented in the computer program SASHA (Small Angle Scattering Shape Determination). It was demonstrated that under certain circumstances a unique envelope can be extracted from the scattering data. This method is only applicable to globular particles with relatively simple shapes and without significant internal cavities. To overcome these limitations, there was another approach developed, which uses different types of Monte-Carlo searches. DALAI_GA is an elegant program, which takes a sphere with diameter equal to the maximum particle size Dmax, which is determined from the scattering data, and fills it with beads. Each bead belongs either to the particle (index=1) or to the solvent (index=0). The shape is thus described by the binary string of length M. Starting from a random string, a genetic algorithm searches for a model that fits the data. Compactness and connectivity constrains are imposed in the search, implemented in the program DAMMIN. If the particle symmetry is known, SASHA and DAMMIN can utilise it as useful constraints. The 'give-n-take' procedure SAXS3D and the program SASMODEL, based on interconnected ellipsoids are ab initio Monte Carlo approaches without limitation in the search space.
An approach that uses an ensemble of Dummy Residues (DRs) and simulated annealing
Simulated annealing
Simulated annealing is a generic probabilistic metaheuristic for the global optimization problem of locating a good approximation to the global optimum of a given function in a large search space. It is often used when the search space is discrete...
to build a locally "chain-compatible" DR-model inside a sphere of diameter Dmax lets one extract more details from SAXS data. This method is implemented in the program GASBOR.
Solution scattering patterns of multi-domain proteins and macromolecular complexes can also be fitted using models built from high resolution (NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
or X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
) structures of individual domains or subunits assuming that their tertiary structure
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
is preserved. Depending on the complexity of the object, different approaches are employed for the global search of the optimum configuration of subunits fitting the experimental data.
Consensus model
The Monte-Carlo based models contain hundreds or thousand parameters, and caution is required to avoid overinterpretation. A common approach is to align a set of models resulting from independent shape reconstruction runs to obtain an average model retaining the most persistent- and conceivably also most reliable-features (e.g. using the program SUPCOMB).Adding missing loops
Disordered surface amino acids ("loops") are frequently unobserved in NMR and crystallographic studies, and may be left missing in the reported models. Such disordered element contribute to the scattering intensity and their probable locations can be found by fixing the known part of the structure and adding the missing parts to fit the SAS pattern from the entire particle. The Dummy Residue approach was extended and the algorithms for adding missing loops or domains were implemented in the program suite CREDO.Hybrid methods
Recently there were a few methods proposed, which use the SAXS data as constraints. The authors aimed to improve results of fold recognition and de novo protein structure predictionDe novo protein structure prediction
In computational biology, de novo protein structure prediction is the task of estimating a protein's tertiary structure from its sequence alone. The problem is very difficult and has occupied leading scientists for decades. Research has focused in three areas: alternate lower-resolution...
methods. SAXS data provide the Fourier transform
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
of the histogram of atomic pair distances (pair distribution function) for a given protein. This can serve as a structural constraint on methods used to determine the native conformational fold of the protein. Threading or fold recognition assumes that 3D structure is more conserved than sequence. Thus, very divergent sequences may have similar structure. Ab initio methods, on the other hand, challenge one of the biggest problems in molecular biology, namely, to predict the folding of a protein "from scratch", using no homologous sequences or structures. Using the "SAXS filter", the authors were able to purify the set of de novo protein models significantly. This was further proved by structure homology
Homology modeling
Homology modeling, also known as comparative modeling of protein refers to constructing an atomic-resolution model of the "target" protein from its amino acid sequence and an experimental three-dimensional structure of a related homologous protein...
searches. It was also shown, that the combination of SAXS scores with scores, used in threading methods, significantly improves the performance of fold recognition. On one example it was demonstrated how approximate tertiary structure of modular proteins can be assembled from high resolution NMR structures of domains, using SAXS data, confining the translational degrees of freedom. Another example shows how the SAXS data can be combined together with NMR, X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
and electron microscopy to reconstruct the quaternary structure of multidomain protein.
Flexible systems
An elegant method to tackle the problem of intrinsically disordered or multi-domain proteins with flexible linkers was proposed recently. It allows coexistence of different conformations of a protein, which together contribute to the average experimental scattering pattern. Initially, EOM (ensemble optimization method) generates a pool of models covering the protein configuration space. The scattering curve is then calculated for each model. In the second step, the program selects subsets of protein models. Average experimental scattering is calculated for each subset and fitted to the experimental SAXS data. If the best fit is not found, models are reshuffled between different subsets and new average scattering calculation and fitting to the experimental data is performed. This method has been tested on two proteins– denaturedDenaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
lysozyme
Lysozyme
Lysozyme, also known as muramidase or N-acetylmuramide glycanhydrolase, are glycoside hydrolases, enzymes that damage bacterial cell walls by catalyzing hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between...
and Bruton's protein kinase
Protein kinase
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them . Phosphorylation usually results in a functional change of the target protein by changing enzyme activity, cellular location, or association with other proteins...
. It gave some interesting and promising results.
Biological molecule layers and GISAS
Coatings of biomolecules can be studied with grazing-incidence X-ray and neutron scattering. IsGISAXS (Grazing Incidence Small Angle X-ray Scattering) is a software program dedicated to the simulation and analysis of GISAXS from nanostructures. IsGISAXS only encompasses the scattering by nanometric sized particles, which are buried in a matrix subsurface or supported on a substrate or buried in a thin layer on a substrate. The case of holes is also handled. The geometry is restricted to a plane of particles. The scattering cross section is decomposed in terms of interference function and particle form factorForm factor
Form factor may refer to:*Form factor or emissivity, the proportion of energy transmitted by that object which can be transferred to another object...
. The emphasis is put on the grazing incidence geometry which induces a "beam refraction effect". The particle form factor is calculated within the Distorted wave Born approximation (DWBA), starting as an unperturbed state with sharp interfaces or with the actual perpendicular profile of refraction index. Various kinds of simple geometrical shapes are available with a full account of size and shape distributions in the Decoupling Approximation (DA), in the Local Monodisperse Approximation (LMA) and also in the Size-Spacing Correlation Approximation (SSCA). Both, disordered systems of particles defined by their particle-particle pair correlation function
Correlation function
A correlation function is the correlation between random variables at two different points in space or time, usually as a function of the spatial or temporal distance between the points...
and bi-dimensional crystal or para-crystal are considered.
See also
- Small-angle X-ray scatteringSmall-angle X-ray scatteringSmall-angle X-ray scattering is a small-angle scattering technique where the elastic scattering of X-rays by a sample which has inhomogeneities in the nm-range, is recorded at very low angles...
(SAXS) - Small angle neutron scattering (SANS)
- Grazing-incidence small-angle X-ray scatteringGrazing-incidence small-angle X-ray scatteringGrazing-incidence small-angle scattering is a scattering technique used to study nanostructured surfaces and thin films. The scattered probe is either photons or neutrons...
(GISAXS) - X-ray crystallographyX-ray crystallographyX-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
- Electron microscopy
- NMRNMRNMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
- Neutron spin echoNeutron spin echoNeutron spin echo spectroscopy is an inelastic neutron scattering technique invented by Ferenc Mezei in the 1970's, and developed in collaboration with John Hayter.In recognition of his work and in other areas, Mezei was awarded the first in 1999....
- Protein Data BankProtein Data BankThe Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
- Protein dynamics
- Protein foldingProtein foldingProtein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
- Protein threading
- Homology modelingHomology modelingHomology modeling, also known as comparative modeling of protein refers to constructing an atomic-resolution model of the "target" protein from its amino acid sequence and an experimental three-dimensional structure of a related homologous protein...
- Rosetta@homeRosetta@homeRosetta@home is a distributed computing project for protein structure prediction on the Berkeley Open Infrastructure for Network Computing platform, run by the Baker laboratory at the University of Washington...
- BrukerBrukerBruker is a leading provider of high-performance scientific instruments and solutions for molecular and materials research, as well as for industrial and applied analysis...
External links
Examples of beamlines to measure biological SAXS- SIBYLS — beamline at Advanced Light SourceAdvanced Light SourceThe Advanced Light Source at Lawrence Berkeley National Laboratory in Berkeley, California is a synchrotron light source. Built from 1987 to 1993, it currently employs 210 scientists and staff. Part of the building in which it is housed was completed in 1942 for a 4.67 m cyclotron, designed by...
, Berkeley, USA - SAXS — beamline at ELETTRA Synchrotron Light LaboratoryElettraELETTRA Synchrotron Light Laboratory is a national synchrotron laboratory located in Basovizza on the outskirts of Trieste, Italy.The facility, available for use by the Italian and international scientific communities, houses several ultra bright light sources, which use the synchrotron and free...
, Trieste, Italy - X33 — beamline at DESYDESYThe DESY is the biggest German research center for particle physics, with sites in Hamburg and Zeuthen....
, Hamburg, Germany - D11A — beamline at Brazilian Synchrotron Light Laboratory
Examples of biological SAXS groups
- Small angle X-ray Scattering Group — at EMBL-Hamburg
SAXS Instrument Manufacturer
- Hecus X-Ray Systems GmbH
- Bruker AXS GmbH
- RigakuRigakuRigaku Corporation is an international manufacturer and distributor of scientific, analytical and industrial instrumentation specializing in X-ray related technologies, including X-ray crystallography, X-ray diffraction , X-ray reflectivity, X-ray fluorescence , automation, cryogenics and X-ray...

