
Organic redox reaction
Encyclopedia
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s. In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
oxidations and reductions are different from ordinary redox reactions because many reactions carry the name but do not actually involve electron transfer in the electrochemical
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
sense of the word .
Following the rules for determining the oxidation number
Oxidation number
In coordination chemistry, the oxidation number of a central atom in a coordination compound is the charge that it would have if all the ligands were removed along with the electron pairs that were shared with the central atom. Oxidation numbers are often confused with oxidation states.The...
for an individual carbon atom leads to
- oxidation number -4 for alkaneAlkaneAlkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s, - oxidation number -2 for alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s, alcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, alkyl halides, amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, - oxidation number 0 for alkyneAlkyneAlkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s, ketoneKetoneIn organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, geminalGeminalIn chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
diolDiolA diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s, - oxidation number +2 for carboxylic acidCarboxylic acidCarboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s, amideAmideIn chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s, chloroformChloroformChloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
and - oxidation number +4 for carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, tetrachloromethane.
Methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
is oxidized to carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
because the oxidation number changes from -4 to +4. Classical reductions include alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
reduction to alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s and classical oxidations include oxidation of alcohol
Alcohol oxidation
Alcohol oxidation is an important organic reaction. Primary alcohols can be oxidized either to aldehydes or to carboxylic acids , while the oxidation of secondary alcohols normally terminates at the ketone stage...
s to aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s. In oxidations electrons are removed and the electron density of a molecule is reduced. In reductions electron density increases when electrons are added to the molecule. This terminology is always centered around the organic compound. For example, it is usual to refer to the reduction of a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
by lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, but not to the oxidation of lithium aluminium hydride by a ketone. Many oxidations involve removal of hydrogen atoms from the organic molecule, and the reverse reduction adds hydrogens to an organic molecule.
Many reactions classified as reductions also appear in other classes. For instance conversion of the ketone to an alcohol by lithium aluminium hydride can be considered a reduction but the hydride is also a good nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
in nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
. Many redox reactions in organic chemistry have coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
involving free radical intermediates. True organic redox chemistry can be found in electrochemical organic synthesis or electrosynthesis
Electrosynthesis
Electrosynthesis in organic chemistry is the synthesis of chemical compounds in a electrochemical cell The main advantage of electrosynthesis over an ordinary redox reaction is avoidance of the potential wasteful other half-reaction and the ability to precisely tune the required potential...
. Examples of organic reactions that can take place in an electrochemical cell
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
are the Kolbe electrolysis
Kolbe electrolysis
Kolbe electrolysis or Kolbe reaction is an organic reaction named after Adolph Wilhelm Hermann Kolbe. The Kolbe reaction is formally a decarboxylative dimerisation and proceeds by a radical reaction mechanism...
In disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
reactions the reactant is both oxidised and reduced in the same chemical reaction forming 2 separate compounds.
Asymmetric catalytic reduction
Asymmetric catalytic reduction
Asymmetric catalytic reduction is the use of various chiral catalysts to reduce a prochiral organic compound to obtain a chiral product. This is one of the several techniques used in chiral synthesis....
s and asymmetric catalytic oxidation
Asymmetric catalytic oxidation
Asymmetric catalytic oxidation is a technique of oxidizing various substrates to give an enantiopure product using a catalyst.-Reactions:*Jacobsen epoxidation of alkenes using manganese-salen complex and NaOCl...
s are important in asymmetric synthesis.
Organic reductions
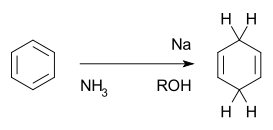
Several reaction mechanisms exist for organic reductions:- Direct electron transfer in one-electron reductionOne-electron reductionA one-electron reduction in organic chemistry involves the transfer of an electron from a metal to an organic substrate. It serves to differentiate between true organic reductions and other reductions such as hydride transfer reactions that actually involve two-electron species.The first...
with the Birch reductionBirch reductionThe Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
as example - HydrideHydrideIn chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
transfer in reductions with for example lithium aluminium hydrideLithium aluminium hydrideLithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
or a hydride shift as in the Meerwein-Ponndorf-Verley reductionMeerwein-Ponndorf-Verley reductionThe Meerwein-Ponndorf-Verley Reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminumalkoxide catalysis in the presence of a sacrificial alcohol... - HydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
reductions with a catalyst such as the Lindlar catalystLindlar catalystA Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate and treated with various forms of lead. The lead additive serves to deactivate the palladium sites. A variety of "catalyst poisons" have been used including lead acetate and lead oxide. The...
or the Adkins catalystAdkins catalystCopper chromite is a complex inorganic composition Cu2Cr2O5, but often containing barium oxide that is used to catalyse certain reactions in organic synthesis. It was first described in 1908. A variety of composition are recognized including Cr2CuO4·CuO·BaCrO4 and Cr2Cu2O5...
or in specific reductions such as the Rosenmund reductionRosenmund reductionThe Rosenmund reduction is a chemical reaction that reduces an acid halide to an aldehyde using hydrogen gas over palladium-on-carbon poisoned with barium sulfate...
. - DisproportionationDisproportionationDisproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
reaction such as the Cannizzaro reactionCannizzaro reactionThe Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position...
Reductions that do not fit in any reduction reaction mechanism and in which just the change in oxidation state is reflected include the Wolff-Kishner reaction.
Organic oxidations
Several reaction mechanisms exist for organic oxidations:- Single electron transfer
- Oxidations through ester intermediates with chromic acidChromic acidThe term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the...
or manganese dioxide - Hydrogen atom transfer as in Free radical halogenationFree radical halogenationIn organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of heat or UV light. The reaction is used for the industrial synthesis of chloroform , dichloromethane , and hexachlorobutadiene...
- Oxidation with oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(combustionCombustionCombustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
) - Oxidation involving ozoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
in ozonolysisOzonolysisOzonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
and peroxidePeroxideA peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
s - Oxidations involving an elimination reactionElimination reactionAn elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
mechanism such as the Swern oxidationSwern oxidationThe Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
, the Kornblum oxidationKornblum oxidationThe Kornblum oxidation is a chemical reaction of a primary halide with dimethyl sulfoxide to form an aldehyde.Like all DMSO-based oxidations, the Kornblum oxidation creates an alkoxysulphonium ion, which, in the presence of a base, such as triethylamine , will eliminate to form the desired aldehyde....
and with reagents such as IBX acid and Dess-Martin periodinaneDess-Martin periodinaneDess–Martin periodinane is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions , shorter reaction times, higher yields, simplified workups,...
. - oxidation by nitroxide radicalsRadical (chemistry)Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
Fremy's saltFremy's saltFrémy's salt, discovered in 1845 by Edmond Frémy , is a chemical compound and a strong oxidizing agent. The formal name is disodium nitrosodisulfonate or Na2NO2, but the expression "Frémy's salt" refers equally well to potassium nitrosodisulfonate, also known as potassium peroxylamine disulfonate...
or TEMPOTEMPOoxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...

