Nucleophilic substitution
Encyclopedia
In organic
and inorganic chemistry
, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile
selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group
; the positive or partially positive atom is referred to as an electrophile
.
The most general form for the reaction may be given as
The electron pair (:) from the nucleophile (Nuc) attacks the substrate (R-LG) forming a new bond, while the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged.
An example of nucleophilic substitution is the hydrolysis
of an alkyl bromide
, R-Br, under alkaline conditions, where the attacking nucleophile is the OH−
and the leaving group
is Br-
.
Nucleophilic substitution reactions are commonplace in organic chemistry, and they can be broadly categorised as taking place at a saturated
aliphatic carbon or at (less often) a saturated aromatic or other unsaturated carbon centre.
 In 1935, Edward D. Hughes and Sir Christopher Ingold
In 1935, Edward D. Hughes and Sir Christopher Ingold
studied nucleophilic substitution reactions of alkyl halides and related compounds. They proposed that there were two main mechanisms at work, both of them competing with each other. The two main mechanisms are the SN1 reaction
and the SN2 reaction
. S stands for chemical substitution, N stands for nucleophilic, and the number represents the kinetic order of the reaction.
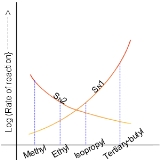
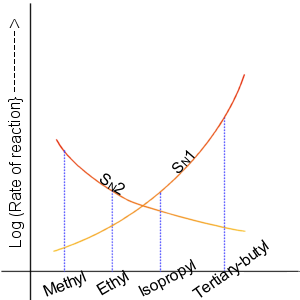
In the SN2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously. SN2 occurs where the central carbon atom is easily accessible to the nucleophile. By contrast the SN1 reaction involves two steps. SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation
.
An example of a substitution reaction taking place by a so-called borderline mechanism as originally studied by Hughes and Ingold is the reaction of 1-phenylethyl chloride with sodium methoxide
in methanol.
The reaction rate
is found to the sum of SN1 and SN2 components with 61% (3,5 M , 70°C) taking place by the latter.
mechanism is observed in reactions of thionyl chloride
with alcohol
s, and it is similar to SN1 except that the nucleophile is delivered from the same side as the leaving group.
Nucleophilic substitutions can be accompanied by an allylic rearrangement
as seen in reactions such as the Ferrier rearrangement
. This type of mechanism is called an SN1' or SN2' reaction (depending on the kinetics). With allyl
ic halides or sulphonates, for example, the nucleophile may attack at the γ unsaturated carbon in place of the carbon bearing the leaving group. This may be seen in the reaction of 1-chloro-2-butene with sodium hydroxide to give a mixture of 2-buten-1-ol and 1-buten-3-ol:
The Sn1CB mechanism
appears in inorganic chemistry
. Competing mechanisms exist.
article.
When the substitution occurs at the carbonyl
group, the acyl
group may undergo nucleophilic acyl substitution
. This is the normal mode of substitution with carboxylic acid
derivatives such as acyl chloride
s, ester
s and amide
s.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and inorganic chemistry
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
; the positive or partially positive atom is referred to as an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
.
The most general form for the reaction may be given as
- Nuc: + R-LG → R-Nuc + LG:
The electron pair (:) from the nucleophile (Nuc) attacks the substrate (R-LG) forming a new bond, while the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged.
An example of nucleophilic substitution is the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of an alkyl bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
, R-Br, under alkaline conditions, where the attacking nucleophile is the OH−
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
and the leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
is Br-
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
.
- R-Br + OH− → R-OH + Br−
Nucleophilic substitution reactions are commonplace in organic chemistry, and they can be broadly categorised as taking place at a saturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
aliphatic carbon or at (less often) a saturated aromatic or other unsaturated carbon centre.
SN1 and SN2 reactions

Christopher Kelk Ingold
Sir Christopher Kelk Ingold FRS was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was responsible for the introduction into mainstream chemistry of concepts such as nucleophile,...
studied nucleophilic substitution reactions of alkyl halides and related compounds. They proposed that there were two main mechanisms at work, both of them competing with each other. The two main mechanisms are the SN1 reaction
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
and the SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
. S stands for chemical substitution, N stands for nucleophilic, and the number represents the kinetic order of the reaction.
In the SN2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously. SN2 occurs where the central carbon atom is easily accessible to the nucleophile. By contrast the SN1 reaction involves two steps. SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
.
An example of a substitution reaction taking place by a so-called borderline mechanism as originally studied by Hughes and Ingold is the reaction of 1-phenylethyl chloride with sodium methoxide
Sodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
in methanol.
The reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
is found to the sum of SN1 and SN2 components with 61% (3,5 M , 70°C) taking place by the latter.
| Nucleophilic substitution at carbon | |
|---|---|
| SN1 mechanism | SN2 mechanism |
| Table 1. Nucleophilic substitutions on RX (an alkyl halide or equivalent) | |||
|---|---|---|---|
| Factor | SN1 SN1 reaction The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular... |
SN2 SN2 reaction The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step... |
Comments |
| Kinetics Chemical kinetics Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition... |
Rate = k[RX] | Rate = k[RX][Nuc] | |
| Primary alkyl | Never unless additional stabilising groups present | Good unless a hindered nucleophile is used | |
| Secondary alkyl | Moderate | Moderate | |
| Tertiary alkyl | Excellent | Never | Elimination Elimination reaction An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism... likely if heated or if strong base used |
| Leaving group Leaving group In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate... |
Important | Important | For halogens, I > Br > Cl >> F |
| Nucleophilicity | Unimportant | Important | |
| Preferred solvent Solvent A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature... |
Polar Chemical polarity In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in... protic Protic Protić is a Serbian surname. It may refer to:* Milorad B. Protić, an astronomer* Miodrag B. Protić, a painter* Stojan Protić, Yugoslav political figure... |
Polar aprotic | |
| Stereochemistry Stereochemistry Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules.... |
Racemisation (+ partial inversion Stereochemistry Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules.... possible) |
Inversion | |
| Rearrangements | Common | Rare | Side reaction |
| Eliminations Elimination reaction An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism... |
Common, especially with basic nucleophiles | Only with heat & basic nucleophiles | Side reaction esp. if heated |
Nucleophilic substitution reactions
There are many reactions in organic chemistry that involve this type of mechanism. Common examples include- Organic reductions with hydrideHydrideIn chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
s, for example
-
- R-X → R-HAlkaneAlkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
using LiAlH4Lithium aluminium hydrideLithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
(SN2)- hydrolysisHydrolysisHydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
reactions such as
- hydrolysis
- R-Br + OH− → R-OHAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
+ Br−BromideA bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
(SN2) or - R-Br + H2O → R-OH + HBrHydrobromic acidHydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a...
(SN1)- Williamson ether synthesisWilliamson ether synthesisThe Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and an alcohol. This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction...
- Williamson ether synthesis
- R-Br + OR'−AlkoxideAn alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
→ R-OR'EtherEthers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
+ Br− (SN2)- The Wenker synthesisWenker synthesisThe Wenker synthesis is an organic reaction converting a beta amino alcohol to an aziridine with the aid of sulfuric acid.The original Wenker synthesis of aziridine itself takes place in two steps...
, a ring-closing reaction of aminoalcohols. - The Finkelstein reactionFinkelstein reactionThe Finkelstein reaction, named for the German chemist Hans Finkelstein , is an SN2 reaction that involves the exchange of one halogen atom for another...
, a halide exchange reaction. Phosphorus nucleophiles appear in the Perkow reactionPerkow reactionThe Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl phosphate and an alkyl halide....
and the Michaelis–Arbuzov reaction. - The Kolbe nitrile synthesisKolbe nitrile synthesisThe Kolbe nitrile synthesis is a method for the preparation of alkyl nitriles by reaction of the corresponding alkylhalide with a metal cyanide . A side product for this reaction is the formation of an isonitrile because the cyanide ion is an ambident nucleophile and according to Kornblum's rule is...
, the reaction of alkyl halides with cyanides.
- The Wenker synthesis
- R-X → R-H
Other mechanisms
Besides SN1 and SN2, other mechanisms are known, although they are less common. The SNiSNi
SNi or Substitution Nucleophilic internal stands for a specific but not often encountered nucleophilic aliphatic substitution reaction mechanism. The name was introduced by Cowdrey et al...
mechanism is observed in reactions of thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
with alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, and it is similar to SN1 except that the nucleophile is delivered from the same side as the leaving group.
Nucleophilic substitutions can be accompanied by an allylic rearrangement
Allylic rearrangement
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution....
as seen in reactions such as the Ferrier rearrangement
Ferrier rearrangement
The Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal . It was discovered by the carbohydrate chemist Robert J...
. This type of mechanism is called an SN1' or SN2' reaction (depending on the kinetics). With allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ic halides or sulphonates, for example, the nucleophile may attack at the γ unsaturated carbon in place of the carbon bearing the leaving group. This may be seen in the reaction of 1-chloro-2-butene with sodium hydroxide to give a mixture of 2-buten-1-ol and 1-buten-3-ol:
- CH3CH=CH-CH2-Cl → CH3CH=CH-CH2-OH + CH3CH(OH)-CH=CH2
The Sn1CB mechanism
Sn1CB mechanism
The SN1CB mechanism describes the pathway by which many metal amine complexes undergo substitution, that is ligand exchange. Typically, the reaction entails reaction of a polyamino metal halide with aqueous base to give the corresponding polyamine metal hydroxide:The rate law for the reaction...
appears in inorganic chemistry
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
. Competing mechanisms exist.
Nucleophilic substitution at unsaturated carbon centres
Nucleophilic substitution via the SN1 or SN2 mechanism does not generally occur with vinyl or aryl halides or related compounds. Under certain conditions nucleophilic substitutions may occur, via other mechanisms such as those described in the nucleophilic aromatic substitutionNucleophilic aromatic substitution
right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
article.
When the substitution occurs at the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group, the acyl
Acyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
group may undergo nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
. This is the normal mode of substitution with carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
derivatives such as acyl chloride
Acyl chloride
In organic chemistry, an acyl chloride is an organic compound with the functional group -CO-Cl. Their formula is usually written RCOCl, where R is a side chain. They are usually considered to be reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride,...
s, ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s and amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s.

