
Heat of formation group additivity
Encyclopedia
Heat of formation group additivity methods in thermochemistry
enable the calculation and prediction of heat of formation of organic compound
s based on additivity. This method was pioneered by S. W. Benson .
s and alkene
s the method works by collecting a large number of experimental heat of formation data (see: Heat of Formation table
) and then divide each molecule up into distinct groups each consisting of a central atom with multiple ligands:
To each group is then assigned an empirical incremental value which is independent on its position inside the molecule and independent of the nature of its neighbors:
The following example illustrates how these values can be derived.
The experimental heat of formation of ethane
is -20.03 kcal/mol and ethane consists of 2 P groups. Likewise propane
(-25.02 kcal/mol) can be written as 2P+S, isobutane
(-32.07) as 3P+T and neopentane
(-40.18 kcal/mol) as 4P+Q. These four equations and 4 unknowns work out to estimations for P (-10.01 kcal/mol), S (-4.99 kcal/mol), T (-2.03 kcal/mol) and Q (-0.12 kcal/mol). Of course the accuracy will increase when the dataset increases.
the data allow the calculation of heat of formation for isomers. For example the pentanes:
The group additivities for alkenes are:
In alkenes the cis isomer is always less stable than the trans isomer by 1.10 kcal/mol.
More group additivity tables exist for a wide range of functional groups.
The Gronert equation reads:
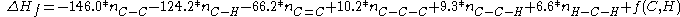
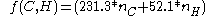
The pentanes are now calculated as:
Key in this treatment is the introduction of 1,3-repulsive and destabilizing interactions and this type of steric hindrance should exist considering the molecular geometry
of simple alkanes. In methane
the distance between the hydrogen atoms is 1.8 angstrom
but the combined van der Waals radii
of hydrogen are 2.4 angstrom implying steric hindrance. Also in propane the methyl to methyl distance is 2.5 angstrom whereas the combined van der Waals radii are much larger (4 angstrom).
In the Gronert model these repulsive 1,3 interactions account for trends in bond dissociation energies
which for example decrease going from methane to ethane to isopropane to neopentane. In this model the homolysis
of a C-H bond releases strain energy
in the alkane. In traditional bonding models the driving force is the ability of alkyl groups to donate electrons to the newly formed free radical carbon.
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
enable the calculation and prediction of heat of formation of organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s based on additivity. This method was pioneered by S. W. Benson .
Benson model
Starting with simple linear and branched alkaneAlkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s and alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s the method works by collecting a large number of experimental heat of formation data (see: Heat of Formation table
Standard enthalpy change of formation (data table)
These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the NIST Chemistry WebBook...
) and then divide each molecule up into distinct groups each consisting of a central atom with multiple ligands:
- X-(A)i(B)j(C)k(D)l
To each group is then assigned an empirical incremental value which is independent on its position inside the molecule and independent of the nature of its neighbors:
- P primary C-(C)(H)3 -10.00
- S secondary C-(C)2(H)2 -5.00
- T tertiary C-(C)3(H) -2.40
- Q quaternary C-(C)4 -0.10
- gaucheGauche (stereochemistry)The term "gauche" refers to conformational isomers where two vicinal groups are separated by a 60° torsion angle. IUPAC defines groups as gauche if they have a "synclinal alignment of groups attached to adjacent atoms"....
correction +0.80 - 1,5 pentane interferencePentane interferencePentane interference or syn-pentane interaction is the steric hindrance that the two terminal methyl groups experience in one of the chemical conformations of n-pentane...
correction +1.60
- in kcal/mol and 298 K
The following example illustrates how these values can be derived.
The experimental heat of formation of ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
is -20.03 kcal/mol and ethane consists of 2 P groups. Likewise propane
Propane
Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
(-25.02 kcal/mol) can be written as 2P+S, isobutane
Isobutane
Isobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,...
(-32.07) as 3P+T and neopentane
Neopentane
Neopentane, also called dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is an extremely flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher...
(-40.18 kcal/mol) as 4P+Q. These four equations and 4 unknowns work out to estimations for P (-10.01 kcal/mol), S (-4.99 kcal/mol), T (-2.03 kcal/mol) and Q (-0.12 kcal/mol). Of course the accuracy will increase when the dataset increases.
the data allow the calculation of heat of formation for isomers. For example the pentanes:
- n-pentane = 2P + 3S = -35 (exp. -35 kcal/mol)
- isopentane = 3P + S + T + 1 gauche correction = -36.6 (exp. -36.7 kcal/mol)
- neopentane = 4P + Q = 40.1 (exp. 40.1 kcal/mol)
The group additivities for alkenes are:
- Cd-(H2) +6.27
- Cd-(C)(D) +8.55
- Cd-(C)2 +10.19
- Cd-(Cd)(H) +6.78
- Cd-(Cd)(C) +8.76
- C-(Cd)(H)3 -10.00
- C-(Cd)(C)(H)2 -4.80
- C-(Cd)(C)2(H) -1.67
- C-(Cd)(C)3 +1.77
- C-(Cd)2(H)2 -4.30
- cis correction +1.10
- alkene gauche correction +0.80
In alkenes the cis isomer is always less stable than the trans isomer by 1.10 kcal/mol.
More group additivity tables exist for a wide range of functional groups.
Gronert model
An alternative model has been developed by S. Gronert based not on breaking molecules into fragments but based on 1,2 and 1,3 interactionsThe Gronert equation reads:
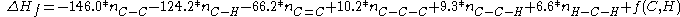
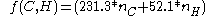
The pentanes are now calculated as:
- n-pentane = 4CC + 12CH + 9HCH + 18HCC + 3CCC + (5C + 12H) = - 35.1 kcal/mol
- isopentane = 4CC + 12CH + 10HCH + 16HCC + 4CCC + (5C + 12H) = - 36.7 kcal/mol
- neopentane = 4CC + 12CH + 12HCH + 12HCC + 6CCC + (5C + 12H) = -40.1 kcal/mol
Key in this treatment is the introduction of 1,3-repulsive and destabilizing interactions and this type of steric hindrance should exist considering the molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
of simple alkanes. In methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
the distance between the hydrogen atoms is 1.8 angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
but the combined van der Waals radii
Van der Waals radius
The van der Waals radius, r, of an atom is the radius of an imaginary hard sphere which can be used to model the atom for many purposes. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms had a finite size and to...
of hydrogen are 2.4 angstrom implying steric hindrance. Also in propane the methyl to methyl distance is 2.5 angstrom whereas the combined van der Waals radii are much larger (4 angstrom).
In the Gronert model these repulsive 1,3 interactions account for trends in bond dissociation energies
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
which for example decrease going from methane to ethane to isopropane to neopentane. In this model the homolysis
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
of a C-H bond releases strain energy
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
in the alkane. In traditional bonding models the driving force is the ability of alkyl groups to donate electrons to the newly formed free radical carbon.

