Amylose
Encyclopedia
Amylose is a linear polymer
made up of D-glucose
units.
This polysaccharide
is one of the two components of starch
, making up approximately 2-30% of the structure. The other component is amylopectin
, which makes up 7-80% of the structure.
Because of its tightly packed structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch
, which has been found to be an effective prebiotic.
of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands.
There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix (A or B form), or it can bind with another hydrophobic guest molecule such as iodine
, a fatty acid
, or an aromatic compound. This is known as the V form and is how amylopectin
binds to amylose to form starch
. Within this group, there are many different variations. Each is notated with V and then a subscript indicating the number of glucose units per turn. The most common is the V6 form, which has six glucose units a turn. V8 and possibly V7 forms exist as well. These provide an even larger space for the guest molecule to bind.
This linear structure can have some rotation around the phi
and psi
angles, but, for the most part, bound glucose ring oxygens lie on one side of the structure. The α(1→4) structure promotes the formation of a helix
structure, making it possible for hydrogen bonds form between the oxygen atoms bound at 2-carbon of one glucose molecule and the 3-carbon of the next glucose molecule.
, amylose is insoluble in water. It also reduces the crystallinity of amylopectin
and how easily water can infiltrate the starch. The higher the amylose content, the less expansion potential and the lower the gel strength for the same starch concentration. This can be countered partially by increasing the granule size.
Fiber X-ray diffraction analysis coupled with computer-based structure refinement has found A-, B-, and C- polymorphs of amylose. Each form corresponds to either the A-, the B-, or the C- starch forms. A- and B- structures have different helical crystal structures and water contents, whereas the C- structure is a mixture of A- and B- unit cells, resulting in an intermediate packing density between the two forms.
; however, because it is more linear than amylopectin, it takes up less space. As a result, it is the preferred starch for storage in plants. It makes up about 30% of the stored starch in plants, though the specific percentage varies by species. The digestive enzyme α-amylase
is responsible for the breakdown of the starch molecule into maltotriose
and maltose
, which can be used as sources of energy.
Amylose is also an important thickener, water binder, emulsion stabilizer, and gelling agent in both industrial and food-based contexts. Loose helical amylose chains have a hydrophobic interior that can bind to hydrophobic molecules such as lipids and aromatic compounds. The one problem with this is that, when it crystallizes or associates, it can lose some stability, often releasing water in the process (syneresis
). When amylose concentration is increased, gel stickiness decreases but gel firmness increases. When other things including amylopectin
bind to amylose, the viscosity
can be affected, but incorporating κ-carrageenan
, alginate, xanthan gum
, or low-molecular-weight sugars can reduce the loss in stability. The ability to bind water can add substance to food, possibly serving as a fat replacement. For example, amylose is responsible for causing white sauce to thicken, but, upon cooling, some separation between the solid and the water will occur.
In a laboratory setting, it can act as a marker. Iodine
molecules fit neatly inside the helical structure
of amylose, binding with the starch polymer that absorbs certain known wavelengths of light. Hence, a common test is the iodine test
for starch. Mix starch with a small amount of yellow iodine solution. In the presence of amylose, a blue-black color will be observed. The intensity of the color can be tested with a colorimeter
, using a red filter to discern the concentration of starch present in the solution. It is also possible to use starch as an indicator
in titrations involving iodine reduction. It is also used in amylose magnetic beads and resin to separate maltose-binding protein
, less sticky long-grain rice, have a much lower glycemic load
, which could be beneficial for diabetics.
Researchers have identified the gene granular binding starch synthase, or GBSS, in potatoes. It is responsible for encoding for the enzyme that directs amylase starch production. If it is inhibited, amylose production will also be interrupted.
Zhong, F., Yokoyama, W.H., Wang, Q., Shoemaker, C.F. 2006. Rice Starch, Amylopectin, and Amylose: Molecular Weight and Solubility in Dimethyl Sulfoxide-Based Solvents. Journal of Agricultural and Food Chemistry. 10.1021:A-G.
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
made up of D-glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
units.
This polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
is one of the two components of starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
, making up approximately 2-30% of the structure. The other component is amylopectin
Amylopectin
Amylopectin is a soluble polysaccharide and highly branched polymer of glucose found in plants. It is one of the two components of starch, the other being amylose.Glucose units are linked in a linear way with α glycosidic bonds...
, which makes up 7-80% of the structure.
Because of its tightly packed structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch
Resistant starch
Resistant starch is starch and starch degradation products that escape digestion in the small intestine of healthy individuals. Resistant starch is considered the third type of dietary fiber, as it can deliver some of the benefits of insoluble fiber and some of the benefits of soluble fiber.Some...
, which has been found to be an effective prebiotic.
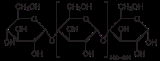
Structure
Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formulaStructural formula
The structural formula of a chemical compound is a graphical representation of the molecular structure, showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly...
of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands.
There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix (A or B form), or it can bind with another hydrophobic guest molecule such as iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, a fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
, or an aromatic compound. This is known as the V form and is how amylopectin
Amylopectin
Amylopectin is a soluble polysaccharide and highly branched polymer of glucose found in plants. It is one of the two components of starch, the other being amylose.Glucose units are linked in a linear way with α glycosidic bonds...
binds to amylose to form starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
. Within this group, there are many different variations. Each is notated with V and then a subscript indicating the number of glucose units per turn. The most common is the V6 form, which has six glucose units a turn. V8 and possibly V7 forms exist as well. These provide an even larger space for the guest molecule to bind.
This linear structure can have some rotation around the phi
Phi
Phi may refer to:In language:*Phi, the Greek letter Φ,φ, the symbol for voiceless bilabial fricativeIn mathematics:*The Golden ratio*Euler's totient function*A statistical measure of association reported with the chi-squared test...
and psi
Psi
-Alphabetic letters:* Psi 23rd letter of the Greek alphabet* Psi , a letter of the early Cyrillic alphabet, adopted from Greek-Mathematics:* Tangential angle of a curve*Chebyshev function*Dedekind psi function*Digamma function...
angles, but, for the most part, bound glucose ring oxygens lie on one side of the structure. The α(1→4) structure promotes the formation of a helix
Helix
A helix is a type of smooth space curve, i.e. a curve in three-dimensional space. It has the property that the tangent line at any point makes a constant angle with a fixed line called the axis. Examples of helixes are coil springs and the handrails of spiral staircases. A "filled-in" helix – for...
structure, making it possible for hydrogen bonds form between the oxygen atoms bound at 2-carbon of one glucose molecule and the 3-carbon of the next glucose molecule.
Physical properties
Unlike amylopectinAmylopectin
Amylopectin is a soluble polysaccharide and highly branched polymer of glucose found in plants. It is one of the two components of starch, the other being amylose.Glucose units are linked in a linear way with α glycosidic bonds...
, amylose is insoluble in water. It also reduces the crystallinity of amylopectin
Amylopectin
Amylopectin is a soluble polysaccharide and highly branched polymer of glucose found in plants. It is one of the two components of starch, the other being amylose.Glucose units are linked in a linear way with α glycosidic bonds...
and how easily water can infiltrate the starch. The higher the amylose content, the less expansion potential and the lower the gel strength for the same starch concentration. This can be countered partially by increasing the granule size.
Fiber X-ray diffraction analysis coupled with computer-based structure refinement has found A-, B-, and C- polymorphs of amylose. Each form corresponds to either the A-, the B-, or the C- starch forms. A- and B- structures have different helical crystal structures and water contents, whereas the C- structure is a mixture of A- and B- unit cells, resulting in an intermediate packing density between the two forms.
Function
Amylose is important in plant energy storage. It is less readily digested than amylopectinAmylopectin
Amylopectin is a soluble polysaccharide and highly branched polymer of glucose found in plants. It is one of the two components of starch, the other being amylose.Glucose units are linked in a linear way with α glycosidic bonds...
; however, because it is more linear than amylopectin, it takes up less space. As a result, it is the preferred starch for storage in plants. It makes up about 30% of the stored starch in plants, though the specific percentage varies by species. The digestive enzyme α-amylase
Amylase
Amylase is an enzyme that catalyses the breakdown of starch into sugars. Amylase is present in human saliva, where it begins the chemical process of digestion. Food that contains much starch but little sugar, such as rice and potato, taste slightly sweet as they are chewed because amylase turns...
is responsible for the breakdown of the starch molecule into maltotriose
Maltotriose
Maltotriose is a trisaccharide consisting of three glucose molecules linked with α-1,4 glycosidic bonds.It is most commonly produced by the digestive enzyme alpha-amylase on amylose in starch...
and maltose
Maltose
Maltose , or malt sugar, is a disaccharide formed from two units of glucose joined with an αbond, formed from a condensation reaction. The isomer "isomaltose" has two glucose molecules linked through an α bond. Maltose is the second member of an important biochemical series of glucose chains....
, which can be used as sources of energy.
Amylose is also an important thickener, water binder, emulsion stabilizer, and gelling agent in both industrial and food-based contexts. Loose helical amylose chains have a hydrophobic interior that can bind to hydrophobic molecules such as lipids and aromatic compounds. The one problem with this is that, when it crystallizes or associates, it can lose some stability, often releasing water in the process (syneresis
Syneresis (chemistry)
Syneresis , in chemistry, is the extraction or expulsion of a liquid from a gel, as when lymph drains from a contracting clot of blood. Another example of syneresis is the collection of whey on the surface of yogurt...
). When amylose concentration is increased, gel stickiness decreases but gel firmness increases. When other things including amylopectin
Amylopectin
Amylopectin is a soluble polysaccharide and highly branched polymer of glucose found in plants. It is one of the two components of starch, the other being amylose.Glucose units are linked in a linear way with α glycosidic bonds...
bind to amylose, the viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
can be affected, but incorporating κ-carrageenan
Carrageenan
Carrageenans or carrageenins are a family of linear sulfated polysaccharides that are extracted from red seaweeds. There are several varieties of carrageen used in cooking and baking. Kappa-carrageenan is used mostly in breading and batter due to its gelling nature...
, alginate, xanthan gum
Xanthan gum
Xanthan gum is a polysaccharide, derived from the bacterial coat of Xanthomonas campestris, used as a food additive and rheology modifier, commonly used as a food thickening agent and a stabilizer...
, or low-molecular-weight sugars can reduce the loss in stability. The ability to bind water can add substance to food, possibly serving as a fat replacement. For example, amylose is responsible for causing white sauce to thicken, but, upon cooling, some separation between the solid and the water will occur.
In a laboratory setting, it can act as a marker. Iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
molecules fit neatly inside the helical structure
Helix
A helix is a type of smooth space curve, i.e. a curve in three-dimensional space. It has the property that the tangent line at any point makes a constant angle with a fixed line called the axis. Examples of helixes are coil springs and the handrails of spiral staircases. A "filled-in" helix – for...
of amylose, binding with the starch polymer that absorbs certain known wavelengths of light. Hence, a common test is the iodine test
Iodine test
The Iodine test is used to test for the presence of starch. Iodine solution — iodine dissolved in an aqueous solution of potassium iodide — reacts with the starch producing a purple black color. The colour can be detected visually with concentrations of iodine as low as 0.00002M at 20°C...
for starch. Mix starch with a small amount of yellow iodine solution. In the presence of amylose, a blue-black color will be observed. The intensity of the color can be tested with a colorimeter
Colorimeter
For articles on Colorimeter see:* Colorimeter * Tristimulus colorimeter...
, using a red filter to discern the concentration of starch present in the solution. It is also possible to use starch as an indicator
Starch indicator
Starch is often used in chemistry as an indicator for redox titrations where triiodide is present. Starch forms a very dark blue-black complex with triiodide which can be made by mixing iodine with iodide . However, the complex is not formed if only iodine or only iodide is present...
in titrations involving iodine reduction. It is also used in amylose magnetic beads and resin to separate maltose-binding protein
Recent studies
High-amylose varieties of riceRice
Rice is the seed of the monocot plants Oryza sativa or Oryza glaberrima . As a cereal grain, it is the most important staple food for a large part of the world's human population, especially in East Asia, Southeast Asia, South Asia, the Middle East, and the West Indies...
, less sticky long-grain rice, have a much lower glycemic load
Glycemic load
The glycemic load is a ranking system for carbohydrate content in food portions based on their glycemic index and a standardized portion size of 100g. Glycemic load or GL combines both the quality and quantity of carbohydrate in one number. It is the best way to predict blood glucose values of...
, which could be beneficial for diabetics.
Researchers have identified the gene granular binding starch synthase, or GBSS, in potatoes. It is responsible for encoding for the enzyme that directs amylase starch production. If it is inhibited, amylose production will also be interrupted.
See also
- amylomaizeAmylomaizeAmylomaize was a term coined by Robert P. Bear of Bear Hybrids Corn Company in Decatur, Illinois to describe his discovery and commercial breeding of a unique cornstarch with high amylose content, also called high amylose starch...
high amylose maize starch
Zhong, F., Yokoyama, W.H., Wang, Q., Shoemaker, C.F. 2006. Rice Starch, Amylopectin, and Amylose: Molecular Weight and Solubility in Dimethyl Sulfoxide-Based Solvents. Journal of Agricultural and Food Chemistry. 10.1021:A-G.
External links
- glycemic load
- http://www.scientificpsychic.com/fitness/carbohydrates1.html
- http://www.gmo-compass.org/eng/glossary/

