
Trypanothione-disulfide reductase
Encyclopedia
In enzymology, a trypanothione-disulfide reductase is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are trypanothione
and NADP+
, whereas its 3 products
are trypanothione disulfide, NADPH
, and H+
.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on a sulfur group of donors with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is trypanothione:NADP+ oxidoreductase. Other names in common use include trypanothione reductase, and NADPH2:trypanothione oxidoreductase. It employs one cofactor
, FAD
.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- trypanothione + NADP+
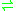 trypanothione disulfide + NADPH + H+
trypanothione disulfide + NADPH + H+
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are trypanothione
Trypanothione
Trypanothione is an unusual form of glutathione containing two molecules of glutathione joined by a spermidine linker. It is found in parasitic protozoa such as leishmania and trypanosomes. These protozoal parasites are the cause of leishmaniasis, sleeping sickness and Chagas' disease....
and NADP+
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
, whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are trypanothione disulfide, NADPH
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
, and H+
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on a sulfur group of donors with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is trypanothione:NADP+ oxidoreductase. Other names in common use include trypanothione reductase, and NADPH2:trypanothione oxidoreductase. It employs one cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
, FAD
FAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
.

