
Riboflavin kinase
Encyclopedia
In enzymology, a riboflavin kinase is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are ATP
and riboflavin
, whereas its two products
are ADP
and FMN
.
Riboflavin is converted into catalytically active cofactors (FAD and FMN) by the actions of riboflavin kinase , which converts it into FMN, and FAD synthetase , which adenylates FMN to FAD. Eukaryotes usually have two separate enzymes, while most prokaryotes have a single bifunctional protein that can carry out both catalyses, although exceptions occur in both cases. While eukaryotic monofunctional riboflavin kinase is orthologous to the bifunctional prokaryotic enzyme
, the monofunctional FAD synthetase differs from its prokaryotic counterpart, and is instead related to the PAPS-reductase family. The bacterial FAD synthetase that is part of the bifunctional enzyme has remote similarity to nucleotidyl transferases and, hence, it may be involved in the adenylylation reaction of FAD synthetases.
This enzyme belongs to the family of transferase
s, to be specific, those transferring phosphorus-containing groups (phosphotransferase
s) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:riboflavin 5'-phosphotransferase. This enzyme is also called flavokinase. This enzyme participates in riboflavin metabolism.
However, archaeal riboflavin kinases are, in general, utilizing CTP
rather than ATP as the donor nucleotide, catalyzing the reaction
Riboflavin kinase can also be isolated from other types of bacteria, all with similar function but a different number of amino acids.
and with NMR
.
The riboflavin kinase enzyme isolated from Thermoplasma acidophilum
contains 220 amino acids. The structure of this enzyme has been determined X-ray crystallography
at a resolution of 2.20 Å. Its secondary structure contains 69 residues (30%) in alpha helix
form, and 60 residues (26%) a beta sheet
conformation. The enzyme contains a magnesium
binding site at amino acids 131 and 133, and a Flavin mononucleotide
binding site at amino acids 188 and 195.
As of late 2007, 14 structures
have been solved for this class of enzymes, with PDB
accession codes , , , , , , , , , , , , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- ATP + riboflavin
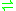 ADP + FMN
ADP + FMN
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
and riboflavin
Riboflavin
Riboflavin, also known as vitamin B2 or additive E101, is an easily absorbed micronutrient with a key role in maintaining health in humans and animals. It is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a...
, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
and FMN
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
.
Riboflavin is converted into catalytically active cofactors (FAD and FMN) by the actions of riboflavin kinase , which converts it into FMN, and FAD synthetase , which adenylates FMN to FAD. Eukaryotes usually have two separate enzymes, while most prokaryotes have a single bifunctional protein that can carry out both catalyses, although exceptions occur in both cases. While eukaryotic monofunctional riboflavin kinase is orthologous to the bifunctional prokaryotic enzyme
Prokaryotic riboflavin biosynthesis protein
In molecular biology, the prokaryotic riboflavin biosynthesis protein is a bifunctional enzyme found in bacteria.Riboflavin is converted into catalytically active cofactors by the actions of riboflavin kinase , which converts it into FMN, and FAD synthetase , which adenylates FMN to FAD...
, the monofunctional FAD synthetase differs from its prokaryotic counterpart, and is instead related to the PAPS-reductase family. The bacterial FAD synthetase that is part of the bifunctional enzyme has remote similarity to nucleotidyl transferases and, hence, it may be involved in the adenylylation reaction of FAD synthetases.
This enzyme belongs to the family of transferase
Transferase
In biochemistry, a transferase is an enzyme that catalyzes the transfer of a functional group from one molecule to another . For example, an enzyme that catalyzed this reaction would be a transferase:In this example, A would be the donor, and B would be the acceptor...
s, to be specific, those transferring phosphorus-containing groups (phosphotransferase
Phosphotransferase
Phosphotransferases are a category of enzymes that catalyze phosphorylation reactions. The general form of the reactions they catalyze is: A—P + B ⇔ B—P + A...
s) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:riboflavin 5'-phosphotransferase. This enzyme is also called flavokinase. This enzyme participates in riboflavin metabolism.
However, archaeal riboflavin kinases are, in general, utilizing CTP
Cytidine triphosphate
Cytidine triphosphate is a pyrimidine nucleoside triphosphate.CTP is a substrate in the synthesis of RNA.CTP is a high-energy molecule equal to ATP, but its role in the organism is more specific than that of ATP....
rather than ATP as the donor nucleotide, catalyzing the reaction
- CTP + riboflavin
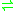 CDP + FMN
CDP + FMN
Riboflavin kinase can also be isolated from other types of bacteria, all with similar function but a different number of amino acids.
Structure
The complete enzyme arrangement can be observed with X-ray crystallographyX-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
and with NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
.
The riboflavin kinase enzyme isolated from Thermoplasma acidophilum
Thermoplasma
In taxonomy, Thermoplasma is a genus of the Thermoplasmataceae.Thermoplasma is a genus of archaea. It belongs to the Thermoplasmata, which thrive in acidic and high-temperature environments. Thermoplasma are facultative anaerobes and respire using sulfur and organic carbon...
contains 220 amino acids. The structure of this enzyme has been determined X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
at a resolution of 2.20 Å. Its secondary structure contains 69 residues (30%) in alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
form, and 60 residues (26%) a beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
conformation. The enzyme contains a magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
binding site at amino acids 131 and 133, and a Flavin mononucleotide
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
binding site at amino acids 188 and 195.
As of late 2007, 14 structures
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , , , , , , , , , and .

