Reichstein process
Encyclopedia
The Reichstein process in chemistry
is a combined chemical
and microbial
method for the production of ascorbic acid
from D-glucose that takes place in several steps. This process was discovered by Nobel Prize
winner Tadeus Reichstein
and his colleagues in 1933 while working in the laboratory of the ETH
in Zürich
.
The microbial oxidation of sorbitol to sorbose is important because it provides the correct stereochemistry
.
in 1935. The first commercially sold vitamin C product was called Cebion from Merck
.
Even today all industrial methods for the production of ascorbic acid are based on the Reichstein process. In modern methods however, sorbose is directly oxidized with a platinum catalyst (developed by Kurt Heyns (1908–2005) in 1942). This method avoids the use of protective groups. A side product with particular modification is 5-Keto-D-gluconic acid.
Novel methods involve genetically modified bacteria.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
is a combined chemical
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
and microbial
Industrial fermentation
Industrial fermentation is the intentional use of fermentation by microorganisms such as bacteria and fungi to make products useful to humans. Fermented products have applications as food as well as in general industry.- Food fermentation :...
method for the production of ascorbic acid
Ascorbic acid
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
from D-glucose that takes place in several steps. This process was discovered by Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
winner Tadeus Reichstein
Tadeus Reichstein
Tadeusz Reichstein was a Polish-born Swiss chemist and Nobel laureate.Reichstein was born into a Jewish family at Włocławek, Congress Poland, and spent his early childhood at Kiev, where his father was an engineer...
and his colleagues in 1933 while working in the laboratory of the ETH
Eth
Eth is a letter used in Old English, Icelandic, Faroese , and Elfdalian. It was also used in Scandinavia during the Middle Ages, but was subsequently replaced with dh and later d. The capital eth resembles a D with a line through the vertical stroke...
in Zürich
Zürich
Zurich is the largest city in Switzerland and the capital of the canton of Zurich. It is located in central Switzerland at the northwestern tip of Lake Zurich...
.
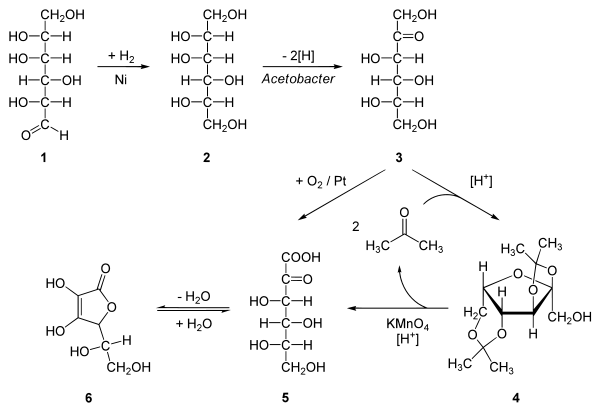
Reaction steps
The reaction steps are:- hydrogenationHydrogenationHydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
of D-glucoseGlucoseGlucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
to D-sorbitolSorbitolSorbitol, also known as glucitol, Sorbogem® and Sorbo®, is a sugar alcohol that the human body metabolizes slowly. It can be obtained by reduction of glucose, changing the aldehyde group to a hydroxyl group. Sorbitol is found in apples, pears, peaches, and prunes...
, an organic reactionOrganic reactionOrganic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
with nickelNickelNickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
as a catalyst under high temperature and high pressure. - Microbial oxidation or fermentationFermentation (biochemistry)Fermentation is the process of extracting energy from the oxidation of organic compounds, such as carbohydrates, using an endogenous electron acceptor, which is usually an organic compound. In contrast, respiration is where electrons are donated to an exogenous electron acceptor, such as oxygen,...
of sorbitol to L-sorboseSorboseSorbose is a ketose belonging to the group of sugars known as monosaccharides. It has a sweetness that is equivalent to sucrose . The commercial production of vitamin C often begins with sorbose. L-Sorbose is the configuration of the naturally occurring sugar....
with acetobacterAcetobacterAcetobacter is a genus of acetic acid bacteria characterized by the ability to convert ethanol to acetic acid in the presence of oxygen. There are several species within this genus, and there are other bacteria capable of forming acetic acid under various conditions; but all of the Acetobacter are...
at pHPHIn chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
4-6 and 30 °C. - protection of the 4 hydroxylAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
groups in sorbose by formation of the acetalAcetalAn acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
with acetoneAcetoneAcetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
and an acidAcidAn acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
to Diacetone-L-sorbose (2,3:4,6−Diisopropyliden−α−L−sorbose) - Organic oxidation with potassium permanganatePotassium permanganatePotassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
followed by heating with water gives the 2-Keto-L-gulonic acid - The final step is a ring-closing step or gamma lactonization with removal of water .
- Intermediate 5 can also be prepared directly from 3 with oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and platinumPlatinumPlatinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
The microbial oxidation of sorbitol to sorbose is important because it provides the correct stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
.
Importance
This process was patented and sold to Hoffmann-La RocheHoffmann-La Roche
F. Hoffmann-La Roche Ltd. is a Swiss global health-care company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX Swiss Exchange....
in 1935. The first commercially sold vitamin C product was called Cebion from Merck
Merck KGaA
Merck KGaA is a German chemical and pharmaceutical company. Merck, also known as “German Merck” and “Merck Darmstadt”, was founded in Darmstadt, Germany, in 1668, making it the world's oldest operating chemical and pharmaceutical company. The company was privately owned until going public in 1995...
.
Even today all industrial methods for the production of ascorbic acid are based on the Reichstein process. In modern methods however, sorbose is directly oxidized with a platinum catalyst (developed by Kurt Heyns (1908–2005) in 1942). This method avoids the use of protective groups. A side product with particular modification is 5-Keto-D-gluconic acid.
Novel methods involve genetically modified bacteria.
Literature
- Boudrant, J. (1990): Microbial processes for ascorbic acid biosynthesis: a review. In: Enzyme Microb Technol. 12(5); 322–9; PMID 1366548;
- Bremus, C. et al. (2006): The use of microorganisms in L-ascorbic acid production. In: J Biotechnol. 124(1); 196–205; PMID 16516325;
External links
- http://www.chemieunterricht.de/dc2/asch2/a-synthe.htm
- http://www.tg.ethz.ch/forschung/projektbeschreib/Baechi/vitamin_c_synthese.htm