Polyproline helix
Encyclopedia
A Polyproline Helix is a type of protein
secondary structure
, which occurs in proteins comprising repeating proline
residues. A left-handed polyproline II helix (PPII, poly-Pro II) is formed when sequential residues all adopt (φ,ψ) backbone dihedral angle
s of roughly (-75°, 150°) and have trans
isomers of their peptide bond
s. Similarly, a more compact right-handed polyproline I helix (PPI, poly-Pro I) is formed when sequential residues all adopt (φ,ψ) backbone dihedral angle
s of roughly (-75°, 160°) and have cis
isomers of their peptide bond
s. Of the twenty common naturally occurring amino acid
s, only proline
is likely to adopt the cis isomer of the peptide bond
, specifically the X-Pro peptide bond; steric and electronic factors heavily favor the trans isomer in most other peptide bonds. However, peptide bond
s that replace proline
with another N-substituted amino acid (such as sarcosine
) are also likely to adopt the cis isomer.
 The PPII helix is defined by (φ,ψ) backbone dihedral angle
The PPII helix is defined by (φ,ψ) backbone dihedral angle
s of roughly (-75°, 150°) and trans isomers of the peptide
bonds. The rotation angle Ω per residue of any polypeptide helix with trans isomers is given by the equation
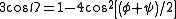
Substitution of the poly-Pro II (φ,ψ) dihedral angles into this equation yields almost exactly Ω = -120°, i.e., the PPII helix is a left-handed helix (since Ω is negative) with three residues per turn (360°/120° = 3). The rise per residue is approximately 3.1 Å. This structure is somewhat similar to that adopted in the fibrous protein collagen
, which is composed mainly of proline, hydroxyproline
, and glycine
. PPII helices are specifically bound by SH3 domain
s; this binding is important for many protein-protein interaction
s and even for interactions between the domains of a single protein.
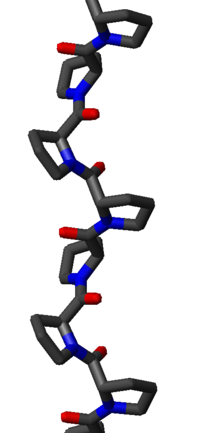 The PPII helix is relatively open and has no internal hydrogen bond
The PPII helix is relatively open and has no internal hydrogen bond
ing, as opposed to the more common helical secondary structure
s, the alpha helix
and its relatives the 310 helix
and the pi helix
, as well as the β-helix
. The amide nitrogen and oxygen atoms are too far apart (approximately 3.8 Å) and oriented incorrectly for hydrogen bonding. Moreover, these atoms are both H-bond acceptors in proline; there is no H-bond donor due to the cyclic side chain.
The PPII backbone dihedral angles (-75°, 150°) are observed frequently in proteins, even for amino acids other than proline
. The Ramachandran plot
is highly populated in the PPII region, comparably to the beta sheet
region around (-135°, 135°). For example, the PPII backbone dihedral angles are often observed in turns
, most commonly in the first residue of a type II β-turn. The "mirror image" PPII backbone dihedral angles (75°, -150°) are rarely seen, except in polymers of the achiral
amino acid glycine
. The analog of the poly-Pro II helix in poly-glycine is called the poly-Gly II helix.
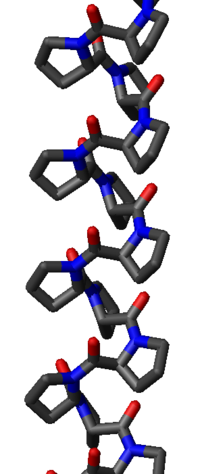 The poly-Pro I helix is much denser than the PPII helix due to the cis isomers of its peptide bond
The poly-Pro I helix is much denser than the PPII helix due to the cis isomers of its peptide bond
s. It is also rarer than the PPII conformation because the cis isomer is higher in energy than the trans. Its typical dihedral angles (-75°, 160°) are close, but not identical to, those of the PPII helix. However, the PPI helix is a right-handed helix and more tightly wound, with roughly 3.3 residues per turn (rather than 3). The rise per residue in the PPI helix is also much smaller, roughly 1.9 Å. Again, there is no internal hydrogen bonding in the poly-Pro I helix, both because an H-bond donor atom is lacking and because the amide nitrogen and oxygen atoms are too distant (roughly 3.8 Å again) and oriented incorrectly.
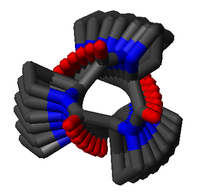
. Interconversions between the PPII and PPI helix forms of poly-proline are slow, due to the high activation energy of X-Pro cis-trans isomerization (Ea ≈ 20 kcal/mol); however, this interconversion may be catalyzed by specific isomerases known as prolyl isomerase
s or PPIases.
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
, which occurs in proteins comprising repeating proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
residues. A left-handed polyproline II helix (PPII, poly-Pro II) is formed when sequential residues all adopt (φ,ψ) backbone dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s of roughly (-75°, 150°) and have trans
Trans
Trans is a Latin noun or prefix, meaning "across", "beyond" or "on the opposite side".Trans may refer to:- Science and technology :* Cis-trans isomerism, in chemistry, a form of stereoisomerism...
isomers of their peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s. Similarly, a more compact right-handed polyproline I helix (PPI, poly-Pro I) is formed when sequential residues all adopt (φ,ψ) backbone dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s of roughly (-75°, 160°) and have cis
Cis
Cis may have the following meanings:* "Cis-" as a prefix of Latin origin, meaning "on the same side [as]" or "on this side [of]", with several derived usages:** In chemistry, cis- refers to cis-trans isomerism...
isomers of their peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s. Of the twenty common naturally occurring amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, only proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
is likely to adopt the cis isomer of the peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
, specifically the X-Pro peptide bond; steric and electronic factors heavily favor the trans isomer in most other peptide bonds. However, peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s that replace proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
with another N-substituted amino acid (such as sarcosine
Sarcosine
Sarcosine, also known as N-methylglycine, is an intermediate and byproduct in glycine synthesis and degradation. Sarcosine is metabolized to glycine by the enzyme sarcosine dehydrogenase, while glycine-N-methyl transferase generates sarcosine from glycine. Sarcosine is a natural amino acid found in...
) are also likely to adopt the cis isomer.
Polyproline II helix

Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s of roughly (-75°, 150°) and trans isomers of the peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
bonds. The rotation angle Ω per residue of any polypeptide helix with trans isomers is given by the equation
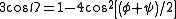
Substitution of the poly-Pro II (φ,ψ) dihedral angles into this equation yields almost exactly Ω = -120°, i.e., the PPII helix is a left-handed helix (since Ω is negative) with three residues per turn (360°/120° = 3). The rise per residue is approximately 3.1 Å. This structure is somewhat similar to that adopted in the fibrous protein collagen
Collagen
Collagen is a group of naturally occurring proteins found in animals, especially in the flesh and connective tissues of mammals. It is the main component of connective tissue, and is the most abundant protein in mammals, making up about 25% to 35% of the whole-body protein content...
, which is composed mainly of proline, hydroxyproline
Hydroxyproline
-4-Hydroxyproline, or L-hydroxyproline , is a common non-proteinogenic amino acid, abbreviated as HYP, e.g., in Protein Data Bank.-Structure and discovery:...
, and glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
. PPII helices are specifically bound by SH3 domain
SH3 domain
The SRC Homology 3 Domain is a small protein domain of about 60 amino acids residues first identified as a conserved sequence in the viral adaptor protein v-Crk and the non-catalytic parts of enzymes such as phospholipase and several cytoplasmic tyrosine kinases such as Abl and Src...
s; this binding is important for many protein-protein interaction
Protein-protein interaction
Protein–protein interactions occur when two or more proteins bind together, often to carry out their biological function. Many of the most important molecular processes in the cell such as DNA replication are carried out by large molecular machines that are built from a large number of protein...
s and even for interactions between the domains of a single protein.

Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing, as opposed to the more common helical secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
s, the alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
and its relatives the 310 helix
3 10 helix
A 310 helix is a type of secondary structure found in proteins.-Structure:The amino acids in a 310-helix are arranged in a right-handed helical structure...
and the pi helix
Pi helix
A pi helix is a type of secondary structure found in proteins. Although thought to be rare, π-helices are actually found in 15% of known protein structures and are believed to be an evolutionary adaptation derived by the insertion of a single amino acid into an α-helix...
, as well as the β-helix
Beta helix
A beta helix is a protein structure formed by the association of parallel beta strands in a helical pattern with either two or three faces. The structure is stabilized by inter-strand hydrogen bonds, protein-protein interactions, and sometimes bound metal ions...
. The amide nitrogen and oxygen atoms are too far apart (approximately 3.8 Å) and oriented incorrectly for hydrogen bonding. Moreover, these atoms are both H-bond acceptors in proline; there is no H-bond donor due to the cyclic side chain.
The PPII backbone dihedral angles (-75°, 150°) are observed frequently in proteins, even for amino acids other than proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
. The Ramachandran plot
Ramachandran plot
-Introduction and early history:A Ramachandran plot , originally developed in 1963 by G. N. Ramachandran C. Ramakrishnan and V...
is highly populated in the PPII region, comparably to the beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
region around (-135°, 135°). For example, the PPII backbone dihedral angles are often observed in turns
Turn (biochemistry)
A turn is an element of secondary structure in proteins where the polypeptide chain reverses its overall direction.- Definition :According to the most common definition, a turn is a structural motif where the Cα atoms of two residues separated by few peptide bonds are in close approach A turn is...
, most commonly in the first residue of a type II β-turn. The "mirror image" PPII backbone dihedral angles (75°, -150°) are rarely seen, except in polymers of the achiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
amino acid glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
. The analog of the poly-Pro II helix in poly-glycine is called the poly-Gly II helix.
Polyproline I helix

Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s. It is also rarer than the PPII conformation because the cis isomer is higher in energy than the trans. Its typical dihedral angles (-75°, 160°) are close, but not identical to, those of the PPII helix. However, the PPI helix is a right-handed helix and more tightly wound, with roughly 3.3 residues per turn (rather than 3). The rise per residue in the PPI helix is also much smaller, roughly 1.9 Å. Again, there is no internal hydrogen bonding in the poly-Pro I helix, both because an H-bond donor atom is lacking and because the amide nitrogen and oxygen atoms are too distant (roughly 3.8 Å again) and oriented incorrectly.

Structural properties
The poly-Pro helices are stable and stiff despite their lack of internal hydrogen bonding, and have been used as a "molecular ruler" in biophysical experiments, e.g., to calibrate distances measured by FRETFluorescence resonance energy transfer
Förster resonance energy transfer , also known as fluorescence resonance energy transfer, resonance energy transfer or electronic energy transfer , is a mechanism describing energy transfer between two chromophores.A donor chromophore, initially in its electronic excited state, may transfer energy...
. Interconversions between the PPII and PPI helix forms of poly-proline are slow, due to the high activation energy of X-Pro cis-trans isomerization (Ea ≈ 20 kcal/mol); however, this interconversion may be catalyzed by specific isomerases known as prolyl isomerase
Prolyl isomerase
Prolyl isomerase is an enzyme found in both prokaryotes and eukaryotes that interconverts the cis and trans isomers of peptide bonds with the amino acid proline. Proline has an unusually conformationally restrained peptide bond due to its cyclic structure with its side chain bonded to its...
s or PPIases.

