
Polyphosphate
Encyclopedia
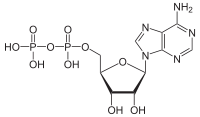
Triphosphates are salts or ester
s of polymeric oxyanion
s formed from tetrahedral PO4 (phosphate
) structural units linked together by sharing oxygen atoms. When two corners are shared the polyphosphate may have a linear chain structure or a cyclic ring structure. In biology the polyphosphate esters AMP
, ADP
and ATP
are involved in energy transfer. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 pm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis and carbohydrate metabolism, respectively.
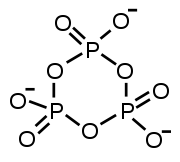
The structure of tripolyphosphoric acid illustrates the principles which define the structures of polyphosphates. It consists of three tetrahedral PO4 units linked together by sharing oxygen atoms. Structurally, the outer tetrahedra share one vertex with the central tetrahedron; the central tetrahedron shares two corners with the others tetrahedra. The corresponding phosphates are related to the acids by loss of the acidic protons. In the case of the cyclic trimer each tetrahedron shares two vertices with adjacent tetrahedra.
Sharing of three corners is possible as in the sheet-structure Phyllosilicates
, but such structures occur only under extreme conditions. Three-corner sharing also occurs in phosphorus pentoxide
, P4O10, which has a 3-dimensional structure.
Chemically, the polymerization reaction can be seen as a condensation reaction. The process begins with two phosphate units coming together.
It is shown as an equilibrium
reaction because it can go in the reverse direction, when it is known as an hydrolysis
reaction because a water molecule is split (Lysed
). The process may continue in steps; at each step another PO3 unit is added to the chain, as indicated by the part in brackets in the illustration of polyphosphoric acid. P3O10 can be seen as the end product of condensation reactions, where each tetrahedron shares three cornes with the others. Conversely, a complex mix of polymers is produces when a small amount of water is added to phosphorus pentoxide.
s. A lone pair of electrons on an oxygen atom can be donated to a hydrogen ion
(proton) or a metal ion in a typical Lewis acid
-Lewis base interaction. This has profound significance in biology. For instance, adenosine triphosphate is about 25% protonated in aqueous solution at pH 7.
Further protonation occurs at lower pH values.
ATP forms chelate
complexes with metal ions. The stability constant
for the equilibrium
is particularly large. The formation of the magnesium complex is a critical element in the process of ATP hydrolysis, as it weakens the link between the terminal phosphate group and the rest of the molecule.
at ΔG -36.8 kJ mol−1 is large by biological standards. Pi stands for inorganic phosphate, which is protonated at biological pH. However, it is not large by inorganic standards. The term "high energy" refers to the fact that it is high relative to the amount of energy released in the organic chemical
-36.8 kJ mol−1 is large by biological standards. Pi stands for inorganic phosphate, which is protonated at biological pH. However, it is not large by inorganic standards. The term "high energy" refers to the fact that it is high relative to the amount of energy released in the organic chemical
reactions that can occur in living systems.
Previously, it was considered either as “molecular fossil” or as only a phosphorus and energy source providing the survival of microorganisms under extreme conditions.
These compounds now known to also have regulatory roles and to occur in representatives of all kingdoms of living organisms, participating in metabolic correction and control on both genetic and enzymatic levels. Polyphosphate is directly involved in the switching-over of the genetic program characteristic of the exponential growth stage of bacteria to the program of cell survival under stationary conditions, “a life in the slow line”. They participate in many regulatory mechanisms occurring in bacteria:
In humans polyphosphates are shown to play a key role in blood coagulation
. Produced and released by platelet
s they activate Factor XII
which is essential for blood clot formation. Furthermore platelets-derived polyphosphates activate blood coagulation factor XII (Hageman factor) that initiates fibrin formation and the generation of a proinflammatory mediator, bradykinin that contributes to leakage from the blood vessels and thrombosis.
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s of polymeric oxyanion
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
s formed from tetrahedral PO4 (phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
) structural units linked together by sharing oxygen atoms. When two corners are shared the polyphosphate may have a linear chain structure or a cyclic ring structure. In biology the polyphosphate esters AMP
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
, ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
and ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
are involved in energy transfer. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 pm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis and carbohydrate metabolism, respectively.
Structure and formation
 |
 |
The structure of tripolyphosphoric acid illustrates the principles which define the structures of polyphosphates. It consists of three tetrahedral PO4 units linked together by sharing oxygen atoms. Structurally, the outer tetrahedra share one vertex with the central tetrahedron; the central tetrahedron shares two corners with the others tetrahedra. The corresponding phosphates are related to the acids by loss of the acidic protons. In the case of the cyclic trimer each tetrahedron shares two vertices with adjacent tetrahedra.
Sharing of three corners is possible as in the sheet-structure Phyllosilicates
Silicate minerals
The silicate minerals make up the largest and most important class of rock-forming minerals, constituting approximately 90 percent of the crust of the Earth. They are classified based on the structure of their silicate group...
, but such structures occur only under extreme conditions. Three-corner sharing also occurs in phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
, P4O10, which has a 3-dimensional structure.
Chemically, the polymerization reaction can be seen as a condensation reaction. The process begins with two phosphate units coming together.
- 2 PO43− + 2 H+ P2O74− + H2O
It is shown as an equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
reaction because it can go in the reverse direction, when it is known as an hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
reaction because a water molecule is split (Lysed
Lysis
Lysis refers to the breaking down of a cell, often by viral, enzymic, or osmotic mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a "lysate"....
). The process may continue in steps; at each step another PO3 unit is added to the chain, as indicated by the part in brackets in the illustration of polyphosphoric acid. P3O10 can be seen as the end product of condensation reactions, where each tetrahedron shares three cornes with the others. Conversely, a complex mix of polymers is produces when a small amount of water is added to phosphorus pentoxide.
Acid-base and complexation properties
Polyphosphates are weak baseBase (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
s. A lone pair of electrons on an oxygen atom can be donated to a hydrogen ion
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
(proton) or a metal ion in a typical Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
-Lewis base interaction. This has profound significance in biology. For instance, adenosine triphosphate is about 25% protonated in aqueous solution at pH 7.
- ATP4- + H+ ATPH3-, pKa
 6.6
6.6
Further protonation occurs at lower pH values.
ATP forms chelate
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
complexes with metal ions. The stability constant
Stability constants of complexes
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex...
for the equilibrium
- ATP4- + Mg2+ MgATP2-, log β
 4
4
is particularly large. The formation of the magnesium complex is a critical element in the process of ATP hydrolysis, as it weakens the link between the terminal phosphate group and the rest of the molecule.
The "high energy" phosphate bond
The energy released in ATP hydrolysis,- ATP4- + H2O → ADP3- +Pi-
at ΔG
 -36.8 kJ mol−1 is large by biological standards. Pi stands for inorganic phosphate, which is protonated at biological pH. However, it is not large by inorganic standards. The term "high energy" refers to the fact that it is high relative to the amount of energy released in the organic chemical
-36.8 kJ mol−1 is large by biological standards. Pi stands for inorganic phosphate, which is protonated at biological pH. However, it is not large by inorganic standards. The term "high energy" refers to the fact that it is high relative to the amount of energy released in the organic chemicalOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
reactions that can occur in living systems.
High-polymeric inorganic polyphosphates
High-polymeric inorganic polyphosphates were found in living organisms by L. Liberman in 1890. These compounds are linear polymers containing a few to several hundred residues of orthophosphate linked by energy-rich phosphoanhydride bonds.Previously, it was considered either as “molecular fossil” or as only a phosphorus and energy source providing the survival of microorganisms under extreme conditions.
These compounds now known to also have regulatory roles and to occur in representatives of all kingdoms of living organisms, participating in metabolic correction and control on both genetic and enzymatic levels. Polyphosphate is directly involved in the switching-over of the genetic program characteristic of the exponential growth stage of bacteria to the program of cell survival under stationary conditions, “a life in the slow line”. They participate in many regulatory mechanisms occurring in bacteria:
- They participate in the induction of rpoS, an RNA-polymerase subunit which is responsible for the expression of a large group of genes involved in adjustments to the stationary growth phase and many stressful agents.
- They are important for cell motility, biofilms formation and virulence.
- Polyphosphates and exopolyphosphataseExopolyphosphataseIn enzymology, an exopolyphosphatase is an enzyme that catalyzes the chemical reactionn + H2O \rightleftharpoons n-1 + phosphate...
s participate in the regulation of the levels of the stringent response factor, guanosine 5'-diphosphate 3'-diphosphate (ppGpp), a second messenger in bacterial cells. - Polyphosphates participate in the formation of channels across the living cell membranes. The above channels formed by polyphosphate and poly-b-hydroxybutyrate with Ca2+ are involved in the transport processes in a variety of organisms.
- An important function of polyphosphate in microorganisms—prokaryotes and the lower eukaryotes—is to handle changing environmental conditions by providing phosphate and energy reserves. Polyphosphates are present in animal cells, and there are many data on its participation in the regulatory processes during development and cellular proliferation and differentiation—especially in bone tissues and brain.
In humans polyphosphates are shown to play a key role in blood coagulation
Coagulation
Coagulation is a complex process by which blood forms clots. It is an important part of hemostasis, the cessation of blood loss from a damaged vessel, wherein a damaged blood vessel wall is covered by a platelet and fibrin-containing clot to stop bleeding and begin repair of the damaged vessel...
. Produced and released by platelet
Platelet
Platelets, or thrombocytes , are small,irregularly shaped clear cell fragments , 2–3 µm in diameter, which are derived from fragmentation of precursor megakaryocytes. The average lifespan of a platelet is normally just 5 to 9 days...
s they activate Factor XII
Factor XII
Coagulation factor XII also known as Hageman factor is a plasma protein. It is the zymogen form of factor XIIa, an enzyme of the serine protease class. In humans, factor XII is encoded by the F12 gene.- Function :...
which is essential for blood clot formation. Furthermore platelets-derived polyphosphates activate blood coagulation factor XII (Hageman factor) that initiates fibrin formation and the generation of a proinflammatory mediator, bradykinin that contributes to leakage from the blood vessels and thrombosis.
See also
- Phosphoric acids and phosphatesPhosphoric acids and PhosphatesThere are various kinds of phosphoric acids and phosphates. Of the many phosphorus oxoacids, the phosphoric acids constitute the largest and most diverse group. The simplest phosphoric acid series begins with monophosphoric acid, continues with many oligophosphoric acids such as diphosphoric acid...
- Sodium trimetaphosphateSodium trimetaphosphateSodium trimetaphosphate , with formula Na3P3O9, is a metaphosphate of sodium. It has the empirical formula NaPO3. It is the sodium salt of trimetaphosphoric acid....
- Sodium hexametaphosphateSodium hexametaphosphateSodium hexametaphosphate is a hexamer of composition 6. Sodium hexametaphosphate of commerce is typically a mixture of polymeric metaphosphates, of which the hexamer is one, and is usually the compound referred to by this name. It is more correctly termed sodium polymetaphosphate. It is prepared...

