Phosphorus pentoxide
Overview
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with molecular formula P
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
4O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
10 (with its common name derived from its empirical formula
Empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms of each element present in a compound. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The empirical formula is used as standard for most ionic...
, P2O5). This white crystalline solid is the anhydride of phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
. It is a powerful desiccant
Desiccant
A desiccant is a hygroscopic substance that induces or sustains a state of dryness in its local vicinity in a moderately well-sealed container....
.
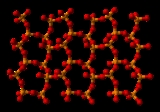
Phosphorus pentoxide crystallizes in at least four forms or polymorphs
Polymorphism (materials science)
Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
. The most familiar one, shown in the figure, comprises molecules of P4O10. Weak van der Waals
Van der Waals
-People:* Fransje van der Waals , Dutch medical physician* Johannes Diderik van der Waals , Dutch physicist-Physics:* the Van der Waals force, named after the physicist* the Van der Waals equation, named after the physicist...
forces hold these molecules together in a hexagonal lattice (However, in spite of the high symmetry of the molecules, the crystal packing is not a close packing).

