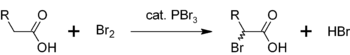
Phosphorus tribromide
Encyclopedia
Phosphorus tribromide is a colourless liquid with the formula P
Br
3. It fumes in air due to hydrolysis
and has a penetrating odour. It is widely used in the laboratory for the conversion of alcohol
s to alkyl bromides.
. An excess of phosphorus is used in order to prevent formation of PBr5:
and PF3
, has both properties of a Lewis base and a Lewis acid
. For example, with a Lewis acid such as boron tribromide
it forms stable 1:1 adducts such as Br3B-PBr3. At the same time PBr3 can react as an electrophile
or Lewis acid in many of its reactions, for example with amine
s.
The most important reaction of PBr3 is with alcohols, where it replaces an OH
group with a bromine atom to produce an alkyl bromide. Note that all three bromines can be transferred.
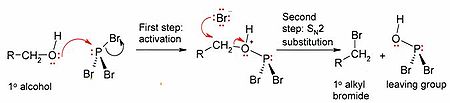
The mechanism (shown for a primary alcohol) involves initial activation of the alcohol oxygen by the electrophilic phosphorus (to form a good leaving group), followed by an SN2 substitution
at the alcohol carbon.
Because of the SN2 substitution step, the reaction generally works well for primary and secondary alcohols, but fails for tertiary alcohols. If the reacting carbon centre is chiral
, the reaction usually occurs with inversion of configuration
at the alcohol alpha carbon
, as is usual with an SN2 reaction.
In a similar reaction, PBr3 also converts carboxylic acid
s to acyl bromide
s.
PBr3 is a reasonably strong reducing agent
, and the oxidation of PBr3 with oxygen
gas is more vigorous than seen with PCl3. It gives an explosive reaction that forms P2O5
and Br2
.
s to alkyl bromides, as described above. PBr3 usually gives higher yields than hydrobromic acid
, and it avoids problems of carbocation
rearrangement- for example even neopentyl bromide can be made from the alcohol in 60% yield.
Another use for PBr3 is as a catalyst for the α-bromination of carboxylic acid
s. Although acyl bromides are rarely made in comparison with acyl chloride
s, they are used as intermediates in Hell-Volhard-Zelinsky halogenation
. Initially PBr3 reacts with the carboxylic acid to form the acyl bromide, which is more reactive towards bromination. The overall process can be represented as
On a commercial scale, phosphorus tribromide is used in the manufacture of pharmaceuticals such as alprazolam
, methohexital
and fenoprofen
. It is also a potent fire suppression agent marketed under the name PhostrEx
.
In reactions that produce phosphorous acid
as a by-product, when working up by distillation be aware that this can decompose above about 160 °C to give phosphine
which can cause explosions in contact with air.
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
Br
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
3. It fumes in air due to hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
and has a penetrating odour. It is widely used in the laboratory for the conversion of alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to alkyl bromides.
Preparation
PBr3 is prepared by treating red phosphorus with bromineBromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
. An excess of phosphorus is used in order to prevent formation of PBr5:
- P4PhosphorusPhosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
+ 6 Br2BromineBromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
→ 4 PBr3
Reactions
Phosphorus tribromide, like PCl3Phosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
and PF3
Phosphorus trifluoride
Phosphorus trifluoride , is a colorless and odorless gas. It is highly toxic and it reacts slowly with water. Its main use is as a ligand in metal complexes...
, has both properties of a Lewis base and a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
. For example, with a Lewis acid such as boron tribromide
Boron tribromide
Boron tribromide, BBr3, is a colorless, fuming liquid compound containing boron and bromine. It is usually made by heating boron trioxide with carbon in the presence of bromine: this generates free boron which reacts vigorously with the bromine...
it forms stable 1:1 adducts such as Br3B-PBr3. At the same time PBr3 can react as an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
or Lewis acid in many of its reactions, for example with amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s.
The most important reaction of PBr3 is with alcohols, where it replaces an OH
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group with a bromine atom to produce an alkyl bromide. Note that all three bromines can be transferred.
- PBr3 + 3 ROHAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
→ 3 RBr + HP(O)(OH)2Phosphorous acidPhosphorous acid is the compound described by the formula H3PO3. This acid is diprotic , not triprotic as might be suggested by this formula. Phosphorous acid is as an intermediate in the preparation of other phosphorus compounds.-Nomenclature and tautomerism:H3PO3 is more clearly described with...
The mechanism (shown for a primary alcohol) involves initial activation of the alcohol oxygen by the electrophilic phosphorus (to form a good leaving group), followed by an SN2 substitution
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
at the alcohol carbon.
Because of the SN2 substitution step, the reaction generally works well for primary and secondary alcohols, but fails for tertiary alcohols. If the reacting carbon centre is chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
, the reaction usually occurs with inversion of configuration
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
at the alcohol alpha carbon
Alpha carbon
The alpha carbon in organic chemistry refers to the first carbon that attaches to a functional group . By extension, the second carbon is the beta carbon, and so on....
, as is usual with an SN2 reaction.
In a similar reaction, PBr3 also converts carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s to acyl bromide
Acyl halide
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
s.
- PBr3 + 3 RCOOHCarboxylic acidCarboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
→ 3 RCOBrAcyl halideAn acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
+ HP(O)(OH)2Phosphorous acidPhosphorous acid is the compound described by the formula H3PO3. This acid is diprotic , not triprotic as might be suggested by this formula. Phosphorous acid is as an intermediate in the preparation of other phosphorus compounds.-Nomenclature and tautomerism:H3PO3 is more clearly described with...
PBr3 is a reasonably strong reducing agent
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, and the oxidation of PBr3 with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
gas is more vigorous than seen with PCl3. It gives an explosive reaction that forms P2O5
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
and Br2
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
.
Applications
The main use for phosphorus tribromide is for conversion of primary or secondary alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to alkyl bromides, as described above. PBr3 usually gives higher yields than hydrobromic acid
Hydrobromic acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a...
, and it avoids problems of carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
rearrangement- for example even neopentyl bromide can be made from the alcohol in 60% yield.
Another use for PBr3 is as a catalyst for the α-bromination of carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s. Although acyl bromides are rarely made in comparison with acyl chloride
Acyl chloride
In organic chemistry, an acyl chloride is an organic compound with the functional group -CO-Cl. Their formula is usually written RCOCl, where R is a side chain. They are usually considered to be reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride,...
s, they are used as intermediates in Hell-Volhard-Zelinsky halogenation
Hell-Volhard-Zelinsky halogenation
The Hell-Volhard-Zelinsky halogenation reaction halogenates carboxylic acids at the α carbon. The reaction is named after three chemists, the German chemists Carl Magnus von Hell and Jacob Volhard and the Russian chemist Nikolay Zelinsky .- Scheme :Unlike other halogenation reactions, this...
. Initially PBr3 reacts with the carboxylic acid to form the acyl bromide, which is more reactive towards bromination. The overall process can be represented as
On a commercial scale, phosphorus tribromide is used in the manufacture of pharmaceuticals such as alprazolam
Alprazolam
Alprazolam is a short-acting anxiolytic of the benzodiazepine class of psychoactive drugs. Alprazolam, like other benzodiazepines, binds to specific sites on the GABAA gamma-amino-butyric acid receptor...
, methohexital
Methohexital
Methohexital, also called methohexitone, is a drug which is a barbiturate derivative. It is classified as short-acting, and has a rapid onset of action...
and fenoprofen
Fenoprofen
Fenoprofen is a non-steroidal anti-inflammatory drug. Fenoprofen calcium is used for symptomatic relief for rheumatoid arthritis, osteoarthritis, and mild to moderate pain...
. It is also a potent fire suppression agent marketed under the name PhostrEx
PhostrEx
PhostrEx is a fire suppression agent developed for use in aviation applications to replace halon, a greenhouse gas . It was developed by Eclipse Aviation for use aboard their Eclipse 500 very light jets as an engine fire suppression system, and is now being marketed to other aviation...
.
Precautions
PBr3 evolves corrosive HBr, is toxic, and reacts violently with water and alcohols.In reactions that produce phosphorous acid
Phosphorous acid
Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic , not triprotic as might be suggested by this formula. Phosphorous acid is as an intermediate in the preparation of other phosphorus compounds.-Nomenclature and tautomerism:H3PO3 is more clearly described with...
as a by-product, when working up by distillation be aware that this can decompose above about 160 °C to give phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
which can cause explosions in contact with air.
Further reading
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.