PDE3 inhibitor
Encyclopedia
Phosphodiesterase
A phosphodiesterase is any enzyme that breaks a phosphodiester bond. Usually, people speaking of phosphodiesterase are referring to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below...
enzyme PDE3
PDE3
PDE3 is a phosphodiesterase. The PDEs belong to at least eleven related gene families, which are different in their primary structure, substrate affinity, responses to effectors, and regulation mechanism....
. They are used for the therapy of acute heart failure and cardiogenic shock
Cardiogenic shock
Cardiogenic shock is based upon an inadequate circulation of blood due to primary failure of the ventricles of the heart to function effectively....
. Approved PDE3 inhibitors include amrinone
Amrinone
Amrinone or inamrinone , trade name Inocor, is a pyridine phosphodiesterase 3 inhibitor. It is a drug that may improve the prognosis in patients with congestive heart failure. Amrinone has been shown to increase the contractions initiated in the heart by high gain calcium induced calcium release...
, cilostazol, milrinone
Milrinone
Milrinone is a phosphodiesterase 3 inhibitor. It potentiates the effect of cyclic adenosine monophosphate .Milrinone also enhances contraction of the left ventricle by increasing Ca2+-ATPase activity on the cardiac sarcoplasmic reticulum...
and enoximone
Enoximone
Enoximone is an imidazole phosphodiesterase inhibitor. It is used in the treatment of congestive heart failure. Selective for phosphodiesterase 3...
. Pimobendane is approved for use in dogs.
Indications
PDE3 inhibitors are indicated as inotropics for the therapy of acute heart failure if catecholamineCatecholamine
Catecholamines are molecules that have a catechol nucleus consisting of benzene with two hydroxyl side groups and a side-chain amine. They include dopamine, as well as the "fight-or-flight" hormones adrenaline and noradrenaline released by the adrenal medulla of the adrenal glands in response to...
s are ineffective. Well controlled studies have shown that these drugs generally increase mortality
Mortality rate
Mortality rate is a measure of the number of deaths in a population, scaled to the size of that population, per unit time...
, so they have to be applied under close observation.
PDE3 inhibitors are also used for the treatment of cardiogenic shock.
Contraindications
ContraindicationContraindication
In medicine, a contraindication is a condition or factor that serves as a reason to withhold a certain medical treatment.Some contraindications are absolute, meaning that there are no reasonable circumstances for undertaking a course of action...
s are severe obstructive cardiomyopathy
Cardiomyopathy
Cardiomyopathy, which literally means "heart muscle disease," is the deterioration of the function of the myocardium for any reason. People with cardiomyopathy are often at risk of arrhythmia or sudden cardiac death or both. Cardiomyopathy can often go undetected, making it especially dangerous to...
, hypovolemia
Hypovolemia
In physiology and medicine, hypovolemia is a state of decreased blood volume; more specifically, decrease in volume of blood plasma...
, tachycardia
Tachycardia
Tachycardia comes from the Greek words tachys and kardia . Tachycardia typically refers to a heart rate that exceeds the normal range for a resting heart rate...
, and ventricular aneurysm
Ventricular aneurysm
Ventricular aneurysms are one of the many complications that may occur after a heart attack . They usually arise from a patch of weakened tissue in a ventricular wall, which swells into a bubble filled with blood. This, in turn, may block the passageways leading out of the heart, leading to...
. Breast feeding is prohibited during treatment.
Adverse effects
The most important adverse effects are arrhythmia, thrombopenia and increased transaminaseTransaminase
In biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
levels.
Mechanism of action
PDE3 inhibitors are a type of phosphodiesterase inhibitorPhosphodiesterase inhibitor
A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase , therefore preventing the inactivation of the intracellular second messengers cyclic adenosine monophosphate and cyclic guanosine monophosphate by the respective PDE...
s. Inhibition of the PDE isoenzyme 3 leads to an increase of intracellular concentrations of the second messenger cyclic adenosine monophosphate
Cyclic adenosine monophosphate
Cyclic adenosine monophosphate is a second messenger important in many biological processes...
(cAMP). cAMP mediates the phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
of protein kinase
Protein kinase
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them . Phosphorylation usually results in a functional change of the target protein by changing enzyme activity, cellular location, or association with other proteins...
s, which in turn activates cardiac calcium channel
Calcium channel
A Calcium channel is an ion channel which displays selective permeability to calcium ions. It is sometimes synonymous as voltage-dependent calcium channel, although there are also ligand-gated calcium channels.-Comparison tables:...
s. An increased calcium influx from the sarcoplasmic reticulum (SR) during phase 2 (the plateau phase) of the cardiac action potential
Cardiac action potential
In electrocardiography, the cardiac action potential is a specialized action potential in the heart, necessary for the electrical conduction system of the heart....
leads to a positive inotropic effect of PDE3 inhibitors: they increase the force of cardiac contraction. Inreased reflux of calcium into the SR following the plateau phase is responsible for their positive lusitropic effect: they increase relaxation speed. Additionally, PDE3 inhibitors act as vasodilators.
First generation PDE3 inhibitors
Recognition that the knowledge about PDE could be used to develop drugs that were PDE inhibitors led to extensive research. Most studies used analogues of the nucleotide substrates or derivatives of natural product inhibitors such as xanthineXanthine
Xanthine , is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine....
(e.g. theophylline
Theophylline
Theophylline, also known as dimethylxanthine, is a methylxanthine drug used in therapy for respiratory diseases such as COPD and asthma under a variety of brand names. Because of its numerous side-effects, the drug is now rarely administered for clinical use. As a member of the xanthine family, it...
) and papaverine
Papaverine
Papaverine is an opium alkaloid antispasmodic drug, used primarily in the treatment of visceral spasm, vasospasm , and occasionally in the treatment of erectile dysfunction...
.
The active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of PDE3 can be considered as a summary of ideas about receptor topography resulting from the first generation inhibitors. The model of the Wells et al. version as cited in Erhardt and Chou (1991) includes the following:
- A phosphatePhosphateA phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
binding area - A lipophilicLipophilicLipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true...
area that accommodates the non-polar side of the riboseRiboseRibose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
moiety - A pyrimidinePyrimidinePyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
binding site - An imidazoleImidazoleImidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
binding site portion of the pyrimidinePyrimidinePyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
binding site - A sterically hindered site
- An area with bulk tolerance
Second generation PDE3 inhibitors
Since selective PDE3 inhibitors were recognised to be cardiotonic drugs there has been great interest in developing new drugs in this category. A large number of heterocyclic compounds have been synthesized during related research. These compounds constitute a second generation of PDE inhibitors. Although they have been directed mostly at PDE3, they present significant structure-activity relationshipStructure-activity relationship
The structure–activity relationship is the relationship between the chemical or 3D structure of a molecule and its biological activity. The analysis of SAR enables the determination of the chemical groups responsible for evoking a target biological effect in the organism...
for the PDEs in general.
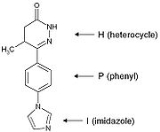
A “heterocycle-phenyl-imidazole” (H-P-I) pattern has been considered to be necessary for positive inotropic activity in cardiac muscle and many second generation inhibitors fit this pattern.
The heterocycle region: Within each heterocycle there is the presence of a dipole and an adjacent acid proton (an amide function). These atoms are believed to mimic the electrophilic center in the phosphate group in cAMP and are confirmed as the primary site of binding. The heterocycle is a transition state analogue inhibitor of PDE. Alkyl groups, limited to either methyl or ethyl
Ethyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
, on the heterocyclic ring usually enhance potency, with occasional exceptions.
The phenyl region: It seems that an electron rich centre, such as phenyl, needs to be present. The beneficial effects of small alkyl groups on the heterocycle could be to twist the central ring away from exact coplanarity with the heterocyclic ring. There is a similar twist in cAMP
Cyclic adenosine monophosphate
Cyclic adenosine monophosphate is a second messenger important in many biological processes...
and there is general agreement that high affinity PDE3 inhibitors should adopt an energetically favoured planar conformation that mimics the anti conformation of cAMP.
The imidazole region: Various substituents have been placed at the para-position of the central phenyl ring. They are electron rich moieties and apparently a positively charged moiety cannot be tolerated in this region of the PDE receptor. There is general agreement about this inhibitor potency: lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
≥ alkyl-CONH- ≥ imidazoyl = pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
in place of the central phenyl with its nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
in the analogous 4 position ≥ alkyl-S- > simple ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
> halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
= amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
> imidazolium (which is totally inactive).
Identification of features common to the most selective inhibitors has led to a “five-point model” with:
- Presence of a strong dipole (carbonylCarbonylIn organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
moiety) at one end of the molecule. - An adjacent acid proton.
- A small sized alkyl substituent on the heterocyclic ring.
- A relatively flat overall topography.
- An electron rich centre and/or a hydrogen bond acceptor site opposite to the dipole.
Examples of selective PDE3 inhibitors
 |
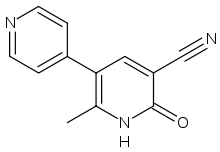
Theophylline is a non-selective agent. In contrast, meribendan is a highly selective inhibitor.
Also, meribendan has a higher level of selectivity in comparison with the parent compound CI-930 because, beside the basic nitrogen adjacent to the lactam moiety it possesses another basic nitrogen (benzimidazole ring), opposite to the primary binding site.

