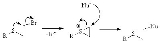
Neighbouring group participation
Encyclopedia
Neighbouring group participation or NGP in organic chemistry
has been defined by IUPAC as the interaction of a reaction centre with a lone pair
of electrons in an atom or the electrons present in a sigma bond
or pi bond
. When NGP is in operation it is normal for the reaction rate
to be increased. It is also possible for the stereochemistry
of the reaction to be abnormal (or unexpected) when compared with a normal reaction. While it is possible for neighbouring groups to influence many reactions in organic chemistry (For instance the reaction of a diene
such as cyclohex-1,3-diene with maleic anhydride
normally gives the endo isomer because of a secondary effect {overlap of the carbonyl group π orbitals with the transition state in the Diels-Alder reaction}) this page is limited to neighbouring group effects seen with carbocation
s and SN2 reactions.
or nitrogen
mustard
with a nucleophile
, the rate of reaction is much higher for the sulfur mustard
and a nucleophile than it would be for a primary alkyl chloride
without a heteroatom
.
 In sugar
In sugar
chemistry anchimeric assistance is an example of NGP.
can stabilize a transition state
by helping to delocalize the positive charge of the carbocation
. For instance the unsaturated
tosylate will react more quickly (1011 times faster for aqueous solvolysis) with a nucleophile than the saturated tosylate.
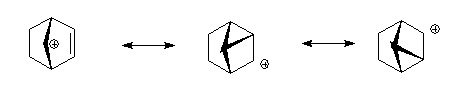
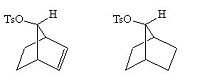 The carbocationic intermediate will be stabilized by resonance where the positive charge is spread over several atoms, in the diagram below this is shown.
The carbocationic intermediate will be stabilized by resonance where the positive charge is spread over several atoms, in the diagram below this is shown.
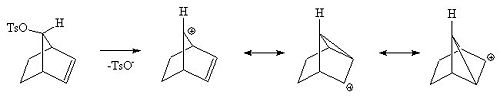 Here is a different view of the same intermediates.
Here is a different view of the same intermediates.
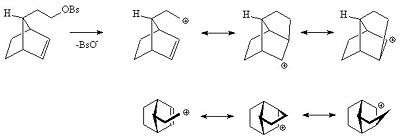
 Even if the alkene is more remote from the reacting center the alkene can still act in this way. For instance in the following alkyl benzenesulfonate the alkene is able to delocalise the carbocation.
Even if the alkene is more remote from the reacting center the alkene can still act in this way. For instance in the following alkyl benzenesulfonate the alkene is able to delocalise the carbocation.
 Also the increase in the rate of the SN2 reaction
Also the increase in the rate of the SN2 reaction
of allyl
bromide with a nucleophile compared with the reaction of n-propyl bromide is because the orbital
s of the π bond overlap with those of the transition state
. In the allyl system the alkene orbitals overlap with the orbitals of a SN2 transition state.
and water
then a mixture of 48% cyclopropylmethyl
alcohol, 47% cyclobutanol
and 5% homoallyl alcohol (but-3-enol) is obtained. This is because the carbocationic intermidate is delocalised onto many different carbons through a reversible ring opening.
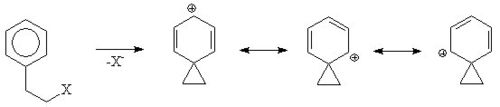
 An aromatic ring can assist in the formation of a carbocation
An aromatic ring can assist in the formation of a carbocation
ic intermediate called a phenonium ion by delocalising the positive charge.
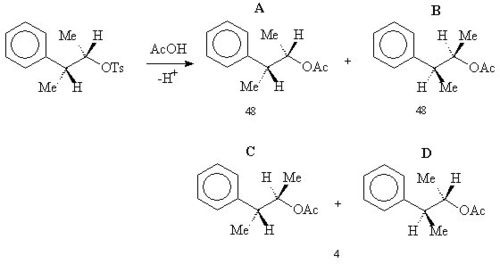
 When the following tosyl
When the following tosyl
ate reacts with acetic acid
in solvolysis
then rather than a simple SN2 reaction forming B, a 48:48:4 mixture of A, B and (C+D) was obtained .
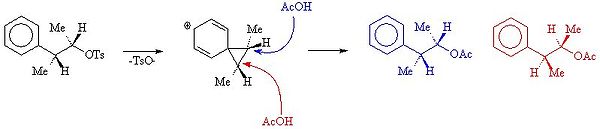
 The mechanism which forms A and B is shown below.
The mechanism which forms A and B is shown below.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
has been defined by IUPAC as the interaction of a reaction centre with a lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
of electrons in an atom or the electrons present in a sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
or pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
. When NGP is in operation it is normal for the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
to be increased. It is also possible for the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of the reaction to be abnormal (or unexpected) when compared with a normal reaction. While it is possible for neighbouring groups to influence many reactions in organic chemistry (For instance the reaction of a diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
such as cyclohex-1,3-diene with maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
normally gives the endo isomer because of a secondary effect {overlap of the carbonyl group π orbitals with the transition state in the Diels-Alder reaction}) this page is limited to neighbouring group effects seen with carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s and SN2 reactions.
NGP by heteroatom lone pairs
A classic example of NGP is the reaction of a sulfurSulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
or nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
mustard
Sulfur mustard
The sulfur mustards, or sulphur mustards, commonly known as mustard gas, are a class of related cytotoxic, vesicant chemical warfare agents with the ability to form large blisters on exposed skin. Pure sulfur mustards are colorless, viscous liquids at room temperature...
with a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
, the rate of reaction is much higher for the sulfur mustard
Sulfur mustard
The sulfur mustards, or sulphur mustards, commonly known as mustard gas, are a class of related cytotoxic, vesicant chemical warfare agents with the ability to form large blisters on exposed skin. Pure sulfur mustards are colorless, viscous liquids at room temperature...
and a nucleophile than it would be for a primary alkyl chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
without a heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
.

Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
chemistry anchimeric assistance is an example of NGP.
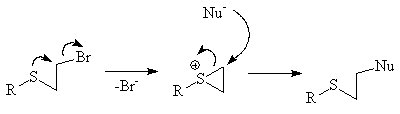
NGP by an alkene
The π orbitals of an alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
can stabilize a transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
by helping to delocalize the positive charge of the carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
. For instance the unsaturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
tosylate will react more quickly (1011 times faster for aqueous solvolysis) with a nucleophile than the saturated tosylate.




SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
of allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
bromide with a nucleophile compared with the reaction of n-propyl bromide is because the orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s of the π bond overlap with those of the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
. In the allyl system the alkene orbitals overlap with the orbitals of a SN2 transition state.

NGP by a cyclopropane, cyclobutane or a homoallyl group
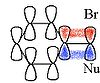
If Cyclopropylmethyl chloride is reacted with ethanolEthanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
then a mixture of 48% cyclopropylmethyl
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
alcohol, 47% cyclobutanol
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
and 5% homoallyl alcohol (but-3-enol) is obtained. This is because the carbocationic intermidate is delocalised onto many different carbons through a reversible ring opening.

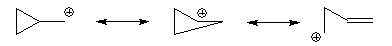
NGP by an aromatic ring
In the case of a phenyl halide the reactivity is higher for the because the SN2 transition state enjoys a similar overlap effect to that in the allyl system.
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
ic intermediate called a phenonium ion by delocalising the positive charge.

Tosyl
A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid...
ate reacts with acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
in solvolysis
Solvolysis
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
then rather than a simple SN2 reaction forming B, a 48:48:4 mixture of A, B and (C+D) was obtained .


External links
- A PhDPHDPHD may refer to:*Ph.D., a doctorate of philosophy*Ph.D. , a 1980s British group*PHD finger, a protein sequence*PHD Mountain Software, an outdoor clothing and equipment company*PhD Docbook renderer, an XML renderer...
thesisThesisA dissertation or thesis is a document submitted in support of candidature for an academic degree or professional qualification presenting the author's research and findings...
written on this subject is at http://alexandria.tue.nl/extra1/PRF2B/7700232.pdf. - IUPAC definition

