
Molecular encapsulation
Encyclopedia
Supramolecular chemistry
Supramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
is the confinement of a guest molecule inside the cavity of a supramolecular host molecule (molecular capsule, molecular container or cage compounds). Examples of supramolecular host molecule include carcerand
Carcerand
A carcerand is a host molecule that completely entraps its guest so that it will not escape even at high temperatures. This type of molecule was first described by Donald J. Cram in 1985 and is derived from the Latin carcer, or prison...
s and endohedral fullerenes
Endohedral fullerenes
Endohedral fullerenes are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex was synthesized in 1985 called La@C60. The @ sign in the name reflects the notion of a small molecule trapped inside a shell...
.
Reactivity of guests
An important implication of encapsulating a molecule at this level is that the guest is prevented from contacting other molecules that it might otherwise react with. Thus the encapsulated molecule behaves very differently from the way it would when in solution. The guest molecule tends to be extremely unreactive and often has much different spectroscopic signatures. Compounds normally highly unstable in solution, such as aryneAryne
In chemistry, an aryne is an uncharged reactive intermediate derived from an aromatic system by removal of two ortho substituents, leaving two orbitals with two electrons distributed between them....
s or cycloheptatetraene have been successfully isolated at room temperature when molecularly encapsulated.
Examples
One of the first examples of encapsulating a structure at the molecular level was demonstrated by CramDonald J. Cram
Donald James Cram was an American chemist who shared the 1987 Nobel Prize in Chemistry with Jean-Marie Lehn and Charles J...
and coworkers in which they were able to isolate highly unstable, antiaromatic cylobutadiene at room temperature by encapsulating it within a hemicarcerand. Isolation of cyclobutadiene allowed chemists to experimentally confirm one of the most fundamental predictions of the rules of aromaticity.
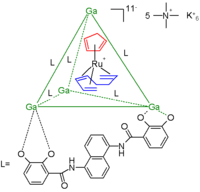
In another example the cage consists of a gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
cluster compound stabilized by 6 bidentate catechol amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s residing at the tetrahedron
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
edges. The guest is a 16 electron
Electron counting
Electron counting is a formalism used for classifying compounds and for explaining or predicting electronic structure and bonding. Many rules in chemistry rely on electron-counting:...
and thus very reactive ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
(a organometallic catalyst) with a cyclopentadienyl
Cyclopentadienyl complex
A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic...
ligand (red) and a 1,3,7-octatriene ligand (blue). The total charge for this anion is 11 and the counterion
Counterion
A counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt the sodium cation is the counterion for the chlorine anion and vice versa.In a charged transition metal complex, a simple A counterion is the ion that accompanies an ionic species in...
s are 5 tetramethyl ammonium cations and 6 potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
cations. The ruthenium compound decomposes in water within minutes but encapsulated it survives in water for weeks.
Other applications:
- the encapsulation of filaments of a self-assemblingMolecular self-assemblyMolecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly, intramolecular self-assembly and intermolecular self-assembly...
bi-copper complexComplex (chemistry)In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
in polymerPolymerA polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
nanowireNanowireA nanowire is a nanostructure, with the diameter of the order of a nanometer . Alternatively, nanowires can be defined as structures that have a thickness or diameter constrained to tens of nanometers or less and an unconstrained length. At these scales, quantum mechanical effects are important —...
s.

