
Metallocene
Encyclopedia
A metallocene is a compound typically consisting of two cyclopentadienyl
anions (Cp, which is C5H5-) bound to a metal center (M) in the oxidation state
II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride
, vanadocene dichloride
. Certain metallocenes and their derivatives exhibit catalytic
properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization
. Metallocenes are a subset of a broader class of organometallic compounds called sandwich compounds.
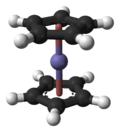
In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromatically
stabilized. Here they are shown in a staggered conformation.
 The general name metallocene is derived from ferrocene
The general name metallocene is derived from ferrocene
, (C5H5)2Fe or Cp2Fe , systematically named bis(η5-cyclopentadienyl
)iron(II). According to the IUPAC definition, a metallocene contains a transition metal
and two cyclopentadienyl ligands coordinated in a sandwich structure, i. e., the two cyclopentadienyl anions are co-planar with equal bond length
s and strengths. Using the nomenclature of "hapticity
", the equivalent bonding of all 5 carbon atoms of a cyclopentadienyl ring is denoted as η5, pronounced "pentahapto." There are exceptions, such as uranocene
, which has two cyclooctatetraene
rings sandwiching a uranium
atom.
In metallocene names, the prefix before the -ocene ending indicates what metallic element is between the Cp groups. For example in ferrocene, iron(II) or ferrous
is present.
In contrast to the more strict definition proposed by IUPAC, which requires a d-block metal and a sandwich structure, the term metallocene and thus the denotation -ocene, is applied in the chemical literature also to non-transition metal compounds, such as Cp2Ba, or structures where the aromatic rings are not co-planar, such as found in manganocene
or titanocene dichloride
(Cp2TiCl2).
Some metallocene complexes of actinides have been reported where there are three cyclopendadienyl ligands for a monometallic complex, all three of them bound η5.
with metal dihalides:
Many other methods have been developed. Chromocene
can be prepared from chromium hexacarbonyl by direct reaction with cyclopentadiene in the presence of diethylamine
; in this case, the formal deprotonation of the cyclopentadiene is followed by reduction
of the resulting protons to hydrogen
gas, facilitating the oxidation of the metal centre.
Metallocenes generally have high thermal stability. Ferrocene can be sublimed in air at over 100 °C with no decomposition; metallocenes are generally purified by vacuum sublimation. Charge-neutral metallocenes are soluble in common organic solvents. Alkyl substituted derivative are particularly soluble, even in alkane solvents.
In metallocenes of the type (C5R5)2M, the cyclopentadienyl rings rotate with very low barriers. Single crystal X-ray diffraction studies reveal both eclipsed or staggered rotamers. For non-substituted metallocenes the energy difference between the staggered and eclipsed conformations is only a few kJ/mol. Crystals of ferrocene and osmocene exhibit eclipsed conformations at low temperatures, whereas in the related bis(pentamethylcyclopentadienyl) complexes the rings usually crystallize in a staggered conformation, apparently to minimize steric hindrance between the methyl group
s.
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
anions (Cp, which is C5H5-) bound to a metal center (M) in the oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride
Titanocene dichloride
Titanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
, vanadocene dichloride
Vanadocene dichloride
Vanadocene dichloride, dichloro bisvanadium is 2VCl2 . It is a structural analoque of titanocene dichloride but with vanadium instead of titanium....
. Certain metallocenes and their derivatives exhibit catalytic
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization
Ziegler-Natta catalyst
A Ziegler–Natta catalyst is a catalyst used in the synthesis of polymers of 1-alkenes . Three types of Ziegler–Natta catalysts are currently employed:* Solid and supported catalysts based on titanium compounds...
. Metallocenes are a subset of a broader class of organometallic compounds called sandwich compounds.
In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromatically
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
stabilized. Here they are shown in a staggered conformation.
Definition

Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
, (C5H5)2Fe or Cp2Fe , systematically named bis(η5-cyclopentadienyl
Cyclopentadienyl complex
A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic...
)iron(II). According to the IUPAC definition, a metallocene contains a transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
and two cyclopentadienyl ligands coordinated in a sandwich structure, i. e., the two cyclopentadienyl anions are co-planar with equal bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s and strengths. Using the nomenclature of "hapticity
Hapticity
The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to...
", the equivalent bonding of all 5 carbon atoms of a cyclopentadienyl ring is denoted as η5, pronounced "pentahapto." There are exceptions, such as uranocene
Uranocene
Uranocene U2 is the most notable cyclooctatetraenide of the f elements and one of the first organouranium compounds to be synthesized. Uranocene is a member of the actinocenes, a group of metallocenes incorporating elements from the actinide series...
, which has two cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
rings sandwiching a uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
atom.
In metallocene names, the prefix before the -ocene ending indicates what metallic element is between the Cp groups. For example in ferrocene, iron(II) or ferrous
Ferrous
Ferrous , in chemistry, indicates a divalent iron compound , as opposed to ferric, which indicates a trivalent iron compound ....
is present.
In contrast to the more strict definition proposed by IUPAC, which requires a d-block metal and a sandwich structure, the term metallocene and thus the denotation -ocene, is applied in the chemical literature also to non-transition metal compounds, such as Cp2Ba, or structures where the aromatic rings are not co-planar, such as found in manganocene
Manganocene
Manganocene or bismanganese is an organometallic compound of manganese. It is a metallocene.In the solid state, manganocene adopts a polymeric structure with every manganese atom coordinated by three cyclopentadienyl ligands, two of which are bridging ligands...
or titanocene dichloride
Titanocene dichloride
Titanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
(Cp2TiCl2).
Some metallocene complexes of actinides have been reported where there are three cyclopendadienyl ligands for a monometallic complex, all three of them bound η5.
Synthesis, properties, and structures of metallocenes
Most metallocenes are prepared by the reaction of sodium cyclopentadienideSodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp or CpNa, where Cp− is the cyclopentadienide anion. Cp is also used as an abbreviation for the cyclopentadienyl ligand in coordination chemistry.-Preparation:Sodium...
with metal dihalides:
- MCl2 + 2 NaC5H5 → M(C5H5)2 + 2 NaCl
Many other methods have been developed. Chromocene
Chromocene
Chromocene is an organochromium compound with the formula Cr2, often abbreviated Cp2Cr. This sandwich compound is structurally similar to ferrocene but does not follow the 18-electron rule because it only has 16 electrons. It also paramagnetic and highly reducing...
can be prepared from chromium hexacarbonyl by direct reaction with cyclopentadiene in the presence of diethylamine
Diethylamine
Diethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3. It is a flammable, strongly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities...
; in this case, the formal deprotonation of the cyclopentadiene is followed by reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of the resulting protons to hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas, facilitating the oxidation of the metal centre.
- Cr(CO)6 + 2 C5H6 → Cr(C5H5)2 + 6 CO + H2
Metallocenes generally have high thermal stability. Ferrocene can be sublimed in air at over 100 °C with no decomposition; metallocenes are generally purified by vacuum sublimation. Charge-neutral metallocenes are soluble in common organic solvents. Alkyl substituted derivative are particularly soluble, even in alkane solvents.
Structure
A structural trend for the series MCp2 involves the variation of the M-C bonds, which elongate as the valence electron count deviates from 18.| M(C5H5)2 | rM-C (pm) | valence electron count |
|---|---|---|
| Fe | 203.3 | 18 |
| Co | 209.6 | 19 |
| Cr | 215.1 | 16 |
| Ni | 218.5 | 20 |
| V | 226 | 15 |
In metallocenes of the type (C5R5)2M, the cyclopentadienyl rings rotate with very low barriers. Single crystal X-ray diffraction studies reveal both eclipsed or staggered rotamers. For non-substituted metallocenes the energy difference between the staggered and eclipsed conformations is only a few kJ/mol. Crystals of ferrocene and osmocene exhibit eclipsed conformations at low temperatures, whereas in the related bis(pentamethylcyclopentadienyl) complexes the rings usually crystallize in a staggered conformation, apparently to minimize steric hindrance between the methyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
s.
Derivatives
- Ansa-metalloceneAnsa-metalloceneAn ansa-metallocene is a type of organometallic compound containing two cyclopentadienyl ligands that are linked by a bridging group such that both cyclopentadienyl groups are bound to the same metal. The link prevents rotation of the cyclopentadienyl ligand and often modifies the structure and...
s: Derivatives of metallocenes include structures with an intramolecular bridge between the two cyclopentadienyl rings (ansa-metallocenes) - Triple decker complexes: compounds with three Cp anions and two metal cations in alternating order, e.g. [Ni2Cp3]+.
- Metallocenium cations: the most famous example is ferrocenium, [Fe(C5H5)2]+, derived from oxidation of ferrocene (few metallocene anions are known).

