_acetate.gif)
Lead(IV) acetate
Encyclopedia
Lead acetate or lead tetraacetate is a chemical compound
with chemical formula
Pb(C2H3O2)4 and is a lead
salt of acetic acid
. It is commercially available often stabilized with acetic acid
.
It can be prepared by reaction of red lead
with acetic acid
The other main lead acetate is lead(II) acetate
.
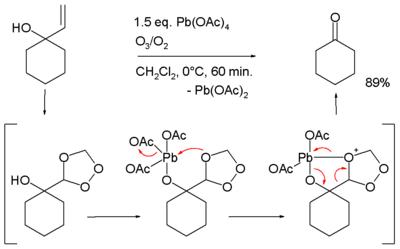
, a source of acetyloxy groups and a general reagent
for the introduction of lead
into organolead compound
s. Some of its many uses in organic chemistry
:
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
Pb(C2H3O2)4 and is a lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
salt of acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. It is commercially available often stabilized with acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
.
It can be prepared by reaction of red lead
Red lead
Lead tetroxide, also called minium, red lead or triplumbic tetroxide, is a bright red or orange crystalline or amorphous pigment. Chemically, red lead is lead tetroxide, Pb3O4, or 2PbO·PbO2....
with acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
The other main lead acetate is lead(II) acetate
Lead(II) acetate
Lead acetate , also known as lead acetate, lead diacetate, plumbous acetate, sugar of lead, lead sugar, salt of Saturn, and Goulard's powder, is a white crystalline chemical compound with a sweetish taste. It is made by treating lead oxide with acetic acid. Like other lead compounds, it is toxic...
.
Reagent in organic chemistry
Lead tetraacetate is a strong oxidizing agentOxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
, a source of acetyloxy groups and a general reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
for the introduction of lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
into organolead compound
Organolead compound
Organolead compounds are chemical compounds containing a chemical bond between carbon and lead. Organolead chemistry is the corresponding science. The first organolead was hexaethyldilead synthesised in 1858. Sharing the same group with carbon, lead is tetravalent.Going down the carbon group the...
s. Some of its many uses in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
:
- acetoxylation of benzylic, allylic and α-oxygen etherEtherEthers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
C-H bonds, for example the photochemical conversion of dioxane to 1,4-dioxene through the 2-acetoxy-1,4-dioxane intermediate and the conversion of α-pinenePinenePinene is a bicyclic monoterpene chemical compound. There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous...
to verbenoneVerbenoneVerbenone is a natural organic compound classified as a terpene which is found naturally in a variety of plants. The chemical has a pleasant characteristic odor. Besides being a natural constituent of plants, it and its analogs are insect pheromones... - oxidation of hydrazoneHydrazoneHydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
s to diazoDiazoDiazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
compounds for example that of hexafluoroacetone hydrazone to bis(trifluoromethyl)diazomethane - aziridineAziridineAziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
formation, for example the reaction of N-aminophthalimide and stilbeneStilbene-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining.... - cleavage of 1,2-diolDiolA diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s to the corresponding aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s or ketoneKetoneIn organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s often replacing ozonolysisOzonolysisOzonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
, for instance the oxidation of di-n-butyl d-tartrateTartrateA tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. Its formula is O−OC-CH-CH-COO− or C4H4O62−.As food additives, tartrates are used as antioxidants, acidity regulators, and emulsifiers...
to n-butyl glyoxylate - reaction with alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s to γ-lactoneLactoneIn chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s - oxidation of alcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s carrying a δ-proton to cyclic etherEtherEthers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s . - Oxidative cleavage of certain allyl alcoholAllyl alcoholAllyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
s in conjunction with ozoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
:

- conversion of acetophenones to phenyl acetic acids

