
Homoserine dehydrogenase
Encyclopedia
In enzymology, a homoserine dehydrogenase is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are L-homoserine, NAD+
, and NADP+
, whereas its 4 products
are L-aspartate 4-semialdehyde, NADH
, NADPH
, and H+
.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is L-homoserine:NAD(P)+ oxidoreductase. Other names in common use include HSDH, and HSD. This enzyme participates in glycine, serine and threonine metabolism and lysine biosynthesis.
Homoserine dehydrogenase catalyses
the third step in the aspartate pathway; the NAD(P)-dependent reduction
of aspartate beta-semialdehyde into homoserine
. Homoserine is an intermediate in the biosynthesis
of threonine
, isoleucine
, and methionine
.
and yeast
, or a bifunctional form consisting of an N-terminal aspartokinase
domain
and a C-terminal homoserine dehydrogenase domain, as found in bacteria such as Escherichia coli
and in plant
s. Structural
analysis of the yeast
monofunctional enzyme indicates that the enzyme is a dimer
composed of three distinct regions; an N-terminal nucleotide-binding domain, a short central dimerisation region, and a C-terminal catalytic domain. The N-terminal domain forms a modified Rossman fold, while the catalytic
domain forms a novel alpha-beta mixed sheet.
As of late 2007, 4 structures
have been solved for this class of enzymes, with PDB
accession codes , , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- L-homoserine + NAD(P)+
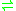 L-aspartate 4-semialdehyde + NAD(P)H + H+
L-aspartate 4-semialdehyde + NAD(P)H + H+
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are L-homoserine, NAD+
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
, and NADP+
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
, whereas its 4 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are L-aspartate 4-semialdehyde, NADH
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
, NADPH
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
, and H+
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is L-homoserine:NAD(P)+ oxidoreductase. Other names in common use include HSDH, and HSD. This enzyme participates in glycine, serine and threonine metabolism and lysine biosynthesis.
Homoserine dehydrogenase catalyses
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the third step in the aspartate pathway; the NAD(P)-dependent reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of aspartate beta-semialdehyde into homoserine
Homoserine
Homoserine is an α-amino acid with the chemical formula HO2CCHCH2CH2OH. L-Homoserine is not one of the common amino acids encoded by DNA. It differs from the proteinogenic amino acid serine by insertion of an additional methylene group...
. Homoserine is an intermediate in the biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
of threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
, and methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
.
Structural studies
The enzyme can be found in a monofunctional form, in some bacteriaBacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
, or a bifunctional form consisting of an N-terminal aspartokinase
Aspartokinase
Aspartokinase is an enzyme that catalyzes the phosphorylation of the amino acid aspartate. This reaction is the first step in the biosynthesis of three essential amino acids: methionine, lysine, and threonine, known as the "aspartate family"...
domain
Protein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
and a C-terminal homoserine dehydrogenase domain, as found in bacteria such as Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
and in plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s. Structural
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
analysis of the yeast
Saccharomyces cerevisiae
Saccharomyces cerevisiae is a species of yeast. It is perhaps the most useful yeast, having been instrumental to baking and brewing since ancient times. It is believed that it was originally isolated from the skin of grapes...
monofunctional enzyme indicates that the enzyme is a dimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
composed of three distinct regions; an N-terminal nucleotide-binding domain, a short central dimerisation region, and a C-terminal catalytic domain. The N-terminal domain forms a modified Rossman fold, while the catalytic
Biocatalysis
Biocatalysis is the use of natural catalysts, such as protein enzymes, to perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task....
domain forms a novel alpha-beta mixed sheet.
As of late 2007, 4 structures
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , and .

