Pentane interference
Encyclopedia
Pentane interference or syn-pentane interaction is the steric hindrance that the two terminal methyl groups experience in one of the chemical conformations of n-pentane. The possible conformations are combinations of anti conformations and gauche conformations and are anti-anti, anti-gauche+, gauche+ - gauche+ and gauche+ - gauche− of which the last one is especially energetically unfavorable. In macromolecule
s such as polyethylene
pentane interference occurs between every fifth carbon atom. This is not to be confused with the 1,3-diaxial interactions of cyclohexane derivatives (gauche interactions shared between substituents and the ring). A clear example of the syn-pentane interaction is apparent in the diaxial versus diequatorial heats of formation of cis 1,3-dialkyl cyclohexanes. Relative to the diequatorial conformer, the diaxial conformer is 2-3 kcal/mol higher in energy than the value that would be expected based on 1,3-diaxial interactions alone. Pentane interference helps explain molecular geometries
in many chemical compounds, product ratios, and purported transition states. One specific type of syn-pentane interaction is known as 1,3 allylic strain or (A1,3 strain).
For instance in certain aldol adducts
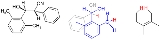
with 2,6-disubstituted aryl
groups the molecular geometry has the vicinal
hydrogen atoms in an antiperiplanar configuration both in a crystal lattice (X-ray diffraction) and in solution
proton (NMR coupling constants) normally reserved for the most bulky groups i.d. both arenes :
 The other contributing factor explaining this conformation is reduction in allylic strain
The other contributing factor explaining this conformation is reduction in allylic strain
by minimizing the dihedral angle
between the arene double bond and the methine
proton.
Pentane interference or syn-pentane interaction is the steric hindrance that the two terminal methyl groups experience in one of the chemical conformations of n-pentane. The possible conformations are combinations of anti conformations and gauche conformations and are anti-anti, anti-gauche+, gauche+ - gauche+ and gauche+ - gauche− of which the last one is especially energetically unfavorable. In macromolecules such as polyethylene pentane interference occurs between every fifth carbon atom. Pentane interference helps explain molecular geometries of many chemical compounds.
For instance in certain aldol adducts with 2,6-disubstituted aryl groups the molecular geometry has the vicinal hydrogen atoms in an antiperiplanar configuration both in a crystal lattice (X-ray diffraction) and in solution proton (NMR coupling constants) normally reserved for the most bulky groups i.d. both arenes [1]:
The other contributing factor explaining this conformation is reduction in allylic strain by minimizing the dihedral angle between the arene double bond and the methine proton.
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
s such as polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
pentane interference occurs between every fifth carbon atom. This is not to be confused with the 1,3-diaxial interactions of cyclohexane derivatives (gauche interactions shared between substituents and the ring). A clear example of the syn-pentane interaction is apparent in the diaxial versus diequatorial heats of formation of cis 1,3-dialkyl cyclohexanes. Relative to the diequatorial conformer, the diaxial conformer is 2-3 kcal/mol higher in energy than the value that would be expected based on 1,3-diaxial interactions alone. Pentane interference helps explain molecular geometries
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
in many chemical compounds, product ratios, and purported transition states. One specific type of syn-pentane interaction is known as 1,3 allylic strain or (A1,3 strain).
For instance in certain aldol adducts
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
with 2,6-disubstituted aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
groups the molecular geometry has the vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
hydrogen atoms in an antiperiplanar configuration both in a crystal lattice (X-ray diffraction) and in solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
proton (NMR coupling constants) normally reserved for the most bulky groups i.d. both arenes :

Allylic strain
thumb | 250 px | right | Allylic strain in an olefin.Allylic strain in organic chemistry is a type of strain energy resulting from the interaction between a substituent on one end of an olefin with an allylic substituent on the other end...
by minimizing the dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
between the arene double bond and the methine
Methine
In chemistry, methine is a trivalent functional group CH, derived formally from methane. The methine group consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen...
proton.
Pentane interference or syn-pentane interaction is the steric hindrance that the two terminal methyl groups experience in one of the chemical conformations of n-pentane. The possible conformations are combinations of anti conformations and gauche conformations and are anti-anti, anti-gauche+, gauche+ - gauche+ and gauche+ - gauche− of which the last one is especially energetically unfavorable. In macromolecules such as polyethylene pentane interference occurs between every fifth carbon atom. Pentane interference helps explain molecular geometries of many chemical compounds.
For instance in certain aldol adducts with 2,6-disubstituted aryl groups the molecular geometry has the vicinal hydrogen atoms in an antiperiplanar configuration both in a crystal lattice (X-ray diffraction) and in solution proton (NMR coupling constants) normally reserved for the most bulky groups i.d. both arenes [1]:
The other contributing factor explaining this conformation is reduction in allylic strain by minimizing the dihedral angle between the arene double bond and the methine proton.

